Oro-Respiratory Dysbiosis and Its Modulatory Effect on Lung Mucosal Toxicity during Exposure or Co-Exposure to Carbon Nanotubes and Cigarette Smoke
- PMID: 38334585
- PMCID: PMC10856953
- DOI: 10.3390/nano14030314
Oro-Respiratory Dysbiosis and Its Modulatory Effect on Lung Mucosal Toxicity during Exposure or Co-Exposure to Carbon Nanotubes and Cigarette Smoke
Abstract
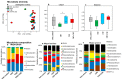
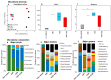
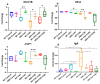
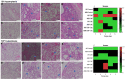
The oro-respiratory microbiome is impacted by inhalable exposures such as smoking and has been associated with respiratory health conditions. However, the effect of emerging toxicants, particularly engineered nanoparticles, alone or in co-exposure with smoking, is poorly understood. Here, we investigated the impact of sub-chronic exposure to carbon nanotube (CNT) particles, cigarette smoke extract (CSE), and their combination. The oral, nasal, and lung microbiomes were characterized using 16S rRNA-based metagenomics. The exposures caused the following shifts in lung microbiota: CNT led to a change from Proteobacteria and Bacteroidetes to Firmicutes and Tenericutes; CSE caused a shift from Proteobacteria to Bacteroidetes; and co-exposure (CNT+CSE) had a mixed effect, maintaining higher numbers of Bacteroidetes (due to the CNT effect) and Tenericutes (due to the CSE effect) compared to the control group. Oral microbiome analysis revealed an abundance of the following genera: Acinetobacter (CNT), Staphylococcus, Aggregatibacter, Allobaculum, and Streptococcus (CSE), and Alkalibacterium (CNT+CSE). These proinflammatory microbial shifts correlated with changes in the relative expression of lung mucosal homeostasis/defense proteins, viz., aquaporin 1 (AQP-1), surfactant protein A (SP-A), mucin 5b (MUC5B), and IgA. Microbiota depletion reversed these perturbations, albeit to a varying extent, confirming the modulatory role of oro-respiratory dysbiosis in lung mucosal toxicity. This is the first demonstration of specific oro-respiratory microbiome constituents as potential modifiers of toxicant effects in exposed lungs.
Keywords: carbon nanotubes; cigarette smoke extract; lung microbiome; nasal microbiome; oral microbiome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Rahman G., Najaf Z., Mehmood A., Bilal S., Shah A.H., Mian S.A., Ali G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. C. 2019;5:3. doi: 10.3390/c5010003. - DOI
-
- Ravichandran P., Baluchamy S., Gopikrishnan R., Biradar S., Ramesh V., Goornavar V., Thomas R., Wilson B.L., Jeffers R., Hall J.C., et al. Pulmonary Biocompatibility Assessment of Inhaled Single-Wall and Multiwall Carbon Nanotubes in BALB/c Mice. J. Biol. Chem. 2011;286:29725–29733. doi: 10.1074/jbc.M111.251884. - DOI - PMC - PubMed
-
- Frank E.A., Carreira V.S., Shanmukhappa K., Medvedovic M., Prows D.R., Yadav J.S. Genetic Susceptibility to Toxicologic Lung Responses among Inbred Mouse Strains Following Exposure to Carbon Nanotubes and Profiling of Underlying Gene Networks. Toxicol. Appl. Pharmacol. 2017;327:59–70. doi: 10.1016/j.taap.2017.04.019. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

