Prospective Approach to Deciphering the Impact of Intercellular Mitochondrial Transfer from Human Neural Stem Cells and Brain Tumor-Initiating Cells to Neighboring Astrocytes
- PMID: 38334595
- PMCID: PMC10854889
- DOI: 10.3390/cells13030204
Prospective Approach to Deciphering the Impact of Intercellular Mitochondrial Transfer from Human Neural Stem Cells and Brain Tumor-Initiating Cells to Neighboring Astrocytes
Abstract
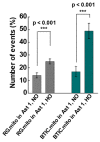
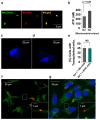
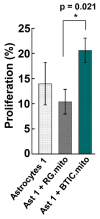
The communication between neural stem cells (NSCs) and surrounding astrocytes is essential for the homeostasis of the NSC niche. Intercellular mitochondrial transfer, a unique communication system that utilizes the formation of tunneling nanotubes for targeted mitochondrial transfer between donor and recipient cells, has recently been identified in a wide range of cell types. Intercellular mitochondrial transfer has also been observed between different types of cancer stem cells (CSCs) and their neighboring cells, including brain CSCs and astrocytes. CSC mitochondrial transfer significantly enhances overall tumor progression by reprogramming neighboring cells. Despite the urgent need to investigate this newly identified phenomenon, mitochondrial transfer in the central nervous system remains largely uncharacterized. In this study, we found evidence of intercellular mitochondrial transfer from human NSCs and from brain CSCs, also known as brain tumor-initiating cells (BTICs), to astrocytes in co-culture experiments. Both NSC and BTIC mitochondria triggered similar transcriptome changes upon transplantation into the recipient astrocytes. In contrast to NSCs, the transplanted mitochondria from BTICs had a significant proliferative effect on the recipient astrocytes. This study forms the basis for mechanistically deciphering the impact of intercellular mitochondrial transfer on recipient astrocytes, which will potentially provide us with new insights into the mechanisms of mitochondrial retrograde signaling.
Keywords: astrocytes; cancer stem cells; intercellular mitochondrial transfer; neural stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pinto G., Saenz-De-Santa-Maria I., Chastagner P., Perthame E., Delmas C., Toulas C., Moyal-Jonathan-Cohen E., Brou C., Zurzolo C. Patient-derived glioblastoma stem cells transfer mitochondria through tunneling nanotubes in tumor organoids. Biochem. J. 2021;478:21–39. doi: 10.1042/BCJ20200710. - DOI - PMC - PubMed
-
- Ferrón S.R., Charalambous M., Radford E., McEwen K., Wildner H., Hind E., Morante-Redolat J.M., Laborda J., Guillemot F., Bauer S.R., et al. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011;475:381–385. doi: 10.1038/nature10229. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

