Blastocyst-Derived Lactic Acid May Regulate S100A6 Expression and Function in Mouse Decidualization via Stimulation of Uterine Epithelial Arachidonic Acid Secretion
- PMID: 38334598
- PMCID: PMC10854550
- DOI: 10.3390/cells13030206
Blastocyst-Derived Lactic Acid May Regulate S100A6 Expression and Function in Mouse Decidualization via Stimulation of Uterine Epithelial Arachidonic Acid Secretion
Abstract
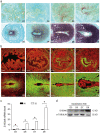
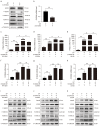
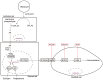
(1) Background: Inflammatory responses are implicated in embryo implantation, decidualization, pregnancy maintenance and labor. Both embryo implantation and decidualization are essential to successful pregnancy in rodents and primates. S100A6 is involved in inflammation, tumor development, apoptosis and calcium homeostasis. S100A6 is strongly expressed in mouse decidua, but the underlying mechanisms of how S100A6 regulates implantation and decidualization are poorly defined. (2) Methods: Mouse endometrial stromal and epithelial cells are isolated from day 4 pseudopregnant mouse uteri. Both immunofluorescence and Western blotting are used to analyze the expression and localization of proteins. The molecular mechanism is verified in vitro by Western blotting and the quantitative polymerase chain reaction. (3) Results: From days 4 to 8 of pregnancy, S100A6 is specifically expressed in mouse subluminal stromal cells. Blastocyst-derived lactic acid induces AA secretion by activating the luminal epithelial p-cPLA2. The epithelial AA induces stromal S100A6 expression through the COX2/PGI2/PPAR δ pathway. Progesterone regulates S100A6 expression through the progesterone receptor (PR). S100A6/RAGE signaling can regulate decidualization via EGFR/ERK1/2 in vitro. (4) Conclusions: S100A6, as an inflammatory mediator, is important for mouse implantation and decidualization.
Keywords: RAGE; S100A6; decidualization; implantation; inflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Zhang T., Zhu W. Early transient suppression of immune checkpoint proteins T-cell immunoglobulin mucin-3 and programmed cell death-1 in peripheral blood lymphocytes after blastocyst transfer is associated with successful implantation. Fertil. Steril. 2020;114:426–435. doi: 10.1016/j.fertnstert.2019.12.022. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

