Deep-Learning-Based Analysis Reveals a Social Behavior Deficit in Mice Exposed Prenatally to Nicotine
- PMID: 38334667
- PMCID: PMC10855062
- DOI: 10.3390/cells13030275
Deep-Learning-Based Analysis Reveals a Social Behavior Deficit in Mice Exposed Prenatally to Nicotine
Abstract
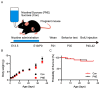
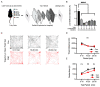
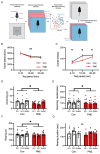
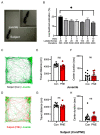
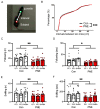
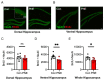
Cigarette smoking during pregnancy is known to be associated with the incidence of attention-deficit/hyperactive disorder (ADHD). Recent developments in deep learning algorithms enable us to assess the behavioral phenotypes of animal models without cognitive bias during manual analysis. In this study, we established prenatal nicotine exposure (PNE) mice and evaluated their behavioral phenotypes using DeepLabCut and SimBA. We optimized the training parameters of DeepLabCut for pose estimation and succeeded in labeling a single-mouse or two-mouse model with high fidelity during free-moving behavior. We applied the trained network to analyze the behavior of the mice and found that PNE mice exhibited impulsivity and a lessened working memory, which are characteristics of ADHD. PNE mice also showed elevated anxiety and deficits in social interaction, reminiscent of autism spectrum disorder (ASD). We further examined PNE mice by evaluating adult neurogenesis in the hippocampus, which is a pathological hallmark of ASD, and demonstrated that newborn neurons were decreased, specifically in the ventral part of the hippocampus, which is reported to be related to emotional and social behaviors. These results support the hypothesis that PNE is a risk factor for comorbidity with ADHD and ASD in mice.
Keywords: ADHD; ASD; DeepLabCut; SimBA; deep learning; prenatal nicotine exposure.
Conflict of interest statement
The authors have no competing interests. We confirm that we have read the journal’s position on issues regarding ethical publication and affirm that this report is consistent with those guidelines.
Figures

References
-
- Office of the Surgeon General (US) Office on Smoking and Health (US) The Health Consequences of Smoking: A Report of the Surgeon General. Centers for Disease Control and Prevention (US); Atlanta, GA, USA: 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

