Human β-Defensin 3 Inhibition of P. gingivalis LPS-Induced IL-1β Production by BV-2 Microglia through Suppression of Cathepsins B and L
- PMID: 38334675
- PMCID: PMC10854704
- DOI: 10.3390/cells13030283
Human β-Defensin 3 Inhibition of P. gingivalis LPS-Induced IL-1β Production by BV-2 Microglia through Suppression of Cathepsins B and L
Abstract
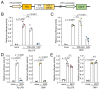
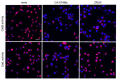
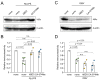
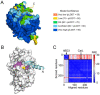
Cathepsin B (CatB) is thought to be essential for the induction of Porphyromonas gingivalis lipopolysaccharide (Pg LPS)-induced Alzheimer's disease-like pathologies in mice, including interleukin-1β (IL-1β) production and cognitive decline. However, little is known about the role of CatB in Pg virulence factor-induced IL-1β production by microglia. We first subjected IL-1β-luciferase reporter BV-2 microglia to inhibitors of Toll-like receptors (TLRs), IκB kinase, and the NLRP3 inflammasome following stimulation with Pg LPS and outer membrane vesicles (OMVs). To clarify the involvement of CatB, we used several known CatB inhibitors, including CA-074Me, ZRLR, and human β-defensin 3 (hBD3). IL-1β production in BV-2 microglia induced by Pg LPS and OMVs was significantly inhibited by the TLR2 inhibitor C29 and the IκB kinase inhibitor wedelolactonne, but not by the NLRPs inhibitor MCC950. Both hBD3 and CA-074Me significantly inhibited Pg LPS-induced IL-1β production in BV-2 microglia. Although CA-074Me also suppressed OMV-induced IL-1β production, hBD3 did not inhibit it. Furthermore, both hBD3 and CA-074Me significantly blocked Pg LPS-induced nuclear NF-κB p65 translocation and IκBα degradation. In contrast, hBD3 and CA-074Me did not block OMV-induced nuclear NF-κB p65 translocation or IκBα degradation. Furthermore, neither ZRLR, a specific CatB inhibitor, nor shRNA-mediated knockdown of CatB expression had any effect on Pg virulence factor-induced IL-1β production. Interestingly, phagocytosis of OMVs by BV-2 microglia induced IL-1β production. Finally, the structural models generated by AlphaFold indicated that hBD3 can bind to the substrate-binding pocket of CatB, and possibly CatL as well. These results suggest that Pg LPS induces CatB/CatL-dependent synthesis and processing of pro-IL-1β without activation of the NLRP3 inflammasome. In contrast, OMVs promote the synthesis and processing of pro-IL-1β through CatB/CatL-independent phagocytic mechanisms. Thus, hBD3 can improve the IL-1β-associated vicious inflammatory cycle induced by microglia through inhibition of CatB/CatL.
Keywords: BV-2 microglia; CA-074Me; Porphyromonas gingivalis; cathepsin B; human β-defensin 3; interleukin-1β; lipopolysaccharide; nuclear factor-κB; outer membrane vesicles.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

