The human OPA1delTTAG mutation induces adult onset and progressive auditory neuropathy in mice
- PMID: 38334784
- PMCID: PMC10858076
- DOI: 10.1007/s00018-024-05115-4
The human OPA1delTTAG mutation induces adult onset and progressive auditory neuropathy in mice
Abstract
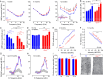
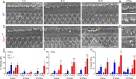
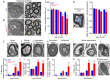
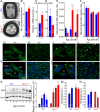
Dominant optic atrophy (DOA) is one of the most prevalent forms of hereditary optic neuropathies and is mainly caused by heterozygous variants in OPA1, encoding a mitochondrial dynamin-related large GTPase. The clinical spectrum of DOA has been extended to a wide variety of syndromic presentations, called DOAplus, including deafness as the main secondary symptom associated to vision impairment. To date, the pathophysiological mechanisms underlying the deafness in DOA remain unknown. To gain insights into the process leading to hearing impairment, we have analyzed the Opa1delTTAG mouse model that recapitulates the DOAplus syndrome through complementary approaches combining morpho-physiology, biochemistry, and cellular and molecular biology. We found that Opa1delTTAG mutation leads an adult-onset progressive auditory neuropathy in mice, as attested by the auditory brainstem response threshold shift over time. However, the mutant mice harbored larger otoacoustic emissions in comparison to wild-type littermates, whereas the endocochlear potential, which is a proxy for the functional state of the stria vascularis, was comparable between both genotypes. Ultrastructural examination of the mutant mice revealed a selective loss of sensory inner hair cells, together with a progressive degeneration of the axons and myelin sheaths of the afferent terminals of the spiral ganglion neurons, supporting an auditory neuropathy spectrum disorder (ANSD). Molecular assessment of cochlea demonstrated a reduction of Opa1 mRNA level by greater than 40%, supporting haploinsufficiency as the disease mechanism. In addition, we evidenced an early increase in Sirtuin 3 level and in Beclin1 activity, and subsequently an age-related mtDNA depletion, increased oxidative stress, mitophagy as well as an impaired autophagic flux. Together, these results support a novel role for OPA1 in the maintenance of inner hair cells and auditory neural structures, addressing new challenges for the exploration and treatment of OPA1-linked ANSD in patients.
Keywords: Deafness; Hereditary optic neuropathy; Hidden hearing loss; Inner ear; Inner hair cell; Mitochondrial homeostasis; Outer hair cell; Retina.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. - DOI - PubMed
-
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. - DOI - PubMed
-
- Lenaers G, Neutzner A, Le Dantec Y, Juschke C, Xiao T, Decembrini S, Swirski S, Kieninger S, Agca C, Kim US, Reynier P, Yu-Wai-Man P, Neidhardt J, Wissinger B. Dominant optic atrophy: culprit mitochondria in the optic nerve. Prog Retin Eye Res. 2020 doi: 10.1016/j.preteyeres.2020.100935. - DOI - PubMed
-
- Amati-Bonneau P, Guichet A, Olichon A, Chevrollier A, Viala F, Miot S, Ayuso C, Odent S, Arrouet C, Verny C, Calmels MN, Simard G, Belenguer P, Wang J, Puel JL, Hamel C, Malthiery Y, Bonneau D, Lenaers G, Reynier P. OPA1 R445H mutation in optic atrophy associated with sensorineural deafness. Ann Neurol. 2005;58:958–963. doi: 10.1002/ana.20681. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

