Exploring the nexus of nuclear receptors in hematological malignancies
- PMID: 38334807
- PMCID: PMC10858172
- DOI: 10.1007/s00018-023-05085-z
Exploring the nexus of nuclear receptors in hematological malignancies
Abstract
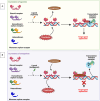
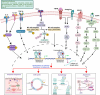
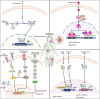
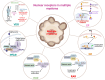
Hematological malignancies (HM) represent a subset of neoplasms affecting the blood, bone marrow, and lymphatic systems, categorized primarily into leukemia, lymphoma, and multiple myeloma. Their prognosis varies considerably, with a frequent risk of relapse despite ongoing treatments. While contemporary therapeutic strategies have extended overall patient survival, they do not offer cures for advanced stages and often lead to challenges such as acquisition of drug resistance, recurrence, and severe side effects. The need for innovative therapeutic targets is vital to elevate both survival rates and patients' quality of life. Recent research has pivoted towards nuclear receptors (NRs) due to their role in modulating tumor cell characteristics including uncontrolled proliferation, differentiation, apoptosis evasion, invasion and migration. Existing evidence emphasizes NRs' critical role in HM. The regulation of NR expression through agonists, antagonists, or selective modulators, contingent upon their levels, offers promising clinical implications in HM management. Moreover, several anticancer agents targeting NRs have been approved by the Food and Drug Administration (FDA). This review highlights the integral function of NRs in HM's pathophysiology and the potential benefits of therapeutically targeting these receptors, suggesting a prospective avenue for more efficient therapeutic interventions against HM.
Keywords: Apoptosis; Differentiation; Hematological malignancies; Homeostasis; Leukemia; Lymphoma; Multiple myeloma; Nuclear receptors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Acute myeloid leukaemia following direct acting antiviral drugs in HCV-infected patients: A 10 years' retrospective single-center study.Clin Res Hepatol Gastroenterol. 2022 Oct;46(8):102000. doi: 10.1016/j.clinre.2022.102000. Epub 2022 Aug 3. Clin Res Hepatol Gastroenterol. 2022. PMID: 35933093
-
Nanomedicines for the treatment of hematological malignancies.J Control Release. 2018 Oct 10;287:194-215. doi: 10.1016/j.jconrel.2018.08.034. Epub 2018 Aug 28. J Control Release. 2018. PMID: 30165140 Review.
-
The rise of apoptosis: targeting apoptosis in hematologic malignancies.Blood. 2018 Sep 20;132(12):1248-1264. doi: 10.1182/blood-2018-02-791350. Epub 2018 Jul 16. Blood. 2018. PMID: 30012635 Review.
-
Deciphering the messages carried by extracellular vesicles in hematological malignancies.Blood Rev. 2021 Mar;46:100734. doi: 10.1016/j.blre.2020.100734. Epub 2020 Jul 14. Blood Rev. 2021. PMID: 32736879 Review.
-
Demystifying the Functional Role of Nuclear Receptors in Esophageal Cancer.Int J Mol Sci. 2022 Sep 19;23(18):10952. doi: 10.3390/ijms231810952. Int J Mol Sci. 2022. PMID: 36142861 Free PMC article. Review.
Cited by
-
Tackling exosome and nuclear receptor interaction: an emerging paradigm in the treatment of chronic diseases.Mil Med Res. 2024 Sep 26;11(1):67. doi: 10.1186/s40779-024-00564-1. Mil Med Res. 2024. PMID: 39327610 Free PMC article. Review.
-
Nuclear receptors in ovarian cancer: changing paradigms in cancer therapeutics.Front Oncol. 2024 Jul 15;14:1383939. doi: 10.3389/fonc.2024.1383939. eCollection 2024. Front Oncol. 2024. PMID: 39077471 Free PMC article. Review.
-
Dynamic interplay of nuclear receptors in tumor cell plasticity and drug resistance: Shifting gears in malignant transformations and applications in cancer therapeutics.Cancer Metastasis Rev. 2024 Mar;43(1):321-362. doi: 10.1007/s10555-024-10171-0. Epub 2024 Mar 22. Cancer Metastasis Rev. 2024. PMID: 38517618 Review.
-
Green synthesis, in silico modeling, and biological evaluation of N-substituted (Z)-5-arylidene imidazolidine/thiazolidine-2,4-dione/4-thione derivatives catalyzed by Bu SO3H core-shell nanostructures.RSC Adv. 2024 Jul 19;14(32):22916-22938. doi: 10.1039/d4ra03881a. eCollection 2024 Jul 19. RSC Adv. 2024. PMID: 39035715 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

