PROTAC-mediated NR4A1 degradation as a novel strategy for cancer immunotherapy
- PMID: 38334978
- PMCID: PMC10857906
- DOI: 10.1084/jem.20231519
PROTAC-mediated NR4A1 degradation as a novel strategy for cancer immunotherapy
Abstract
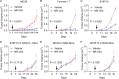
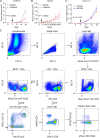
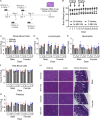
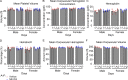
An effective cancer therapy requires killing cancer cells and targeting the tumor microenvironment (TME). Searching for molecules critical for multiple cell types in the TME, we identified NR4A1 as one such molecule that can maintain the immune suppressive TME. Here, we establish NR4A1 as a valid target for cancer immunotherapy and describe a first-of-its-kind proteolysis-targeting chimera (PROTAC, named NR-V04) against NR4A1. NR-V04 degrades NR4A1 within hours in vitro and exhibits long-lasting NR4A1 degradation in tumors with an excellent safety profile. NR-V04 inhibits and frequently eradicates established tumors. At the mechanistic level, NR-V04 induces the tumor-infiltrating (TI) B cells and effector memory CD8+ T (Tem) cells and reduces monocytic myeloid-derived suppressor cells (m-MDSC), all of which are known to be clinically relevant immune cell populations in human melanomas. Overall, NR-V04-mediated NR4A1 degradation holds promise for enhancing anticancer immune responses and offers a new avenue for treating various types of cancers such as melanoma.
© 2024 Wang et al.
Conflict of interest statement
Disclosures: L. Wang reported grants from Sanofi during the conduct of the study; in addition, L. Wang had a patent to Modulators of Nuclear Receptor Subfamily 4 Group A Member 1 (NR4A1) and Uses Thereof issued. D.R. Shaffer reported being an employee of Sanofi, a global healthcare and pharmaceutical company, where he receives a salary and stock awards for his work. K.S.M. Smalley reported grants from Revolution Medicines outside the submitted work. D. Zhou reported grants from Sanofi during the conduct of the study; in addition, D. Zhou had a patent to US Patent Application No: 18/023,696 pending. G. Zheng reported a patent to WO2022/072094 pending. W. Zhang reported grants from Sanofi during the conduct of the study; in addition, W. Zhang had a patent to US 2023/0330237 A1 issued. No other disclosures were reported.
Figures







Update of
-
Unleashing the Power of NR4A1 Degradation as a Novel Strategy for Cancer Immunotherapy.bioRxiv [Preprint]. 2023 Aug 13:2023.08.09.552650. doi: 10.1101/2023.08.09.552650. bioRxiv. 2023. Update in: J Exp Med. 2024 Mar 4;221(3):e20231519. doi: 10.1084/jem.20231519. PMID: 37609171 Free PMC article. Updated. Preprint.
References
-
- Carelock, M.E., Master R.P., Kim M.-C., Jin Z., Wang L., Maharjan C.K., Jua N., Kolb R., Xiao Y., Liao D., et al. 2023. Targeting intracellular proteins with cell type-specific functions for cancer immunotherapy. Life Med. 2:Inad019. 10.1093/lifemedi/lnad019 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

