SARS-CoV-2 Nsp1 cooperates with initiation factors EIF1 and 1A to selectively enhance translation of viral RNA
- PMID: 38335237
- PMCID: PMC10903962
- DOI: 10.1371/journal.ppat.1011535
SARS-CoV-2 Nsp1 cooperates with initiation factors EIF1 and 1A to selectively enhance translation of viral RNA
Abstract
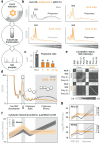
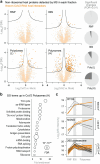
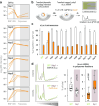
A better mechanistic understanding of virus-host dependencies can help reveal vulnerabilities and identify opportunities for therapeutic intervention. Of particular interest are essential interactions that enable production of viral proteins, as those could target an early step in the virus lifecycle. Here, we use subcellular proteomics, ribosome profiling analyses and reporter assays to detect changes in protein synthesis dynamics during SARS-CoV-2 (CoV2) infection. We identify specific translation factors and molecular chaperones that are used by CoV2 to promote the synthesis and maturation of its own proteins. These can be targeted to inhibit infection, without major toxicity to the host. We also find that CoV2 non-structural protein 1 (Nsp1) cooperates with initiation factors EIF1 and 1A to selectively enhance translation of viral RNA. When EIF1/1A are depleted, more ribosomes initiate translation from a conserved upstream CUG start codon found in all genomic and subgenomic viral RNAs. This results in higher translation of an upstream open reading frame (uORF1) and lower translation of the main ORF, altering the stoichiometry of viral proteins and attenuating infection. Replacing the upstream CUG with AUG strongly inhibits translation of the main ORF independently of Nsp1, EIF1, or EIF1A. Taken together, our work describes multiple dependencies of CoV2 on host biosynthetic networks and proposes a model for dosage control of viral proteins through Nsp1-mediated control of translation start site selection.
Copyright: © 2024 Aviner et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

