ONC201 (Dordaviprone) in Recurrent H3 K27M-Mutant Diffuse Midline Glioma
- PMID: 38335473
- PMCID: PMC11095894
- DOI: 10.1200/JCO.23.01134
ONC201 (Dordaviprone) in Recurrent H3 K27M-Mutant Diffuse Midline Glioma
Abstract
Purpose: Histone 3 (H3) K27M-mutant diffuse midline glioma (DMG) has a dismal prognosis with no established effective therapy beyond radiation. This integrated analysis evaluated single-agent ONC201 (dordaviprone), a first-in-class imipridone, in recurrent H3 K27M-mutant DMG.
Methods: Fifty patients (pediatric, n = 4; adult, n = 46) with recurrent H3 K27M-mutant DMG who received oral ONC201 monotherapy in four clinical trials or one expanded access protocol were included. Eligible patients had measurable disease by Response Assessment in Neuro-Oncology (RANO) high-grade glioma (HGG) criteria and performance score (PS) ≥60 and were ≥90 days from radiation; pontine and spinal tumors were ineligible. The primary end point was overall response rate (ORR) by RANO-HGG criteria. Secondary end points included duration of response (DOR), time to response (TTR), corticosteroid response, PS response, and ORR by RANO low-grade glioma (LGG) criteria. Radiographic end points were assessed by dual-reader, blinded independent central review.
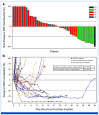
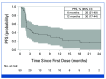
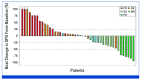
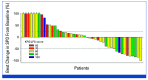
Results: The ORR (RANO-HGG) was 20.0% (95% CI, 10.0 to 33.7). The median TTR was 8.3 months (range, 1.9-15.9); the median DOR was 11.2 months (95% CI, 3.8 to not reached). The ORR by combined RANO-HGG/LGG criteria was 30.0% (95% CI, 17.9 to 44.6). A ≥50% corticosteroid dose reduction occurred in 7 of 15 evaluable patients (46.7% [95% CI, 21.3 to 73.4]); PS improvement occurred in 6 of 34 evaluable patients (20.6% [95% CI, 8.7 to 37.9]). Grade 3 treatment-related treatment-emergent adverse events (TR-TEAEs) occurred in 20.0% of patients; the most common was fatigue (n = 5; 10%); no grade 4 TR-TEAEs, deaths, or discontinuations occurred.
Conclusion: ONC201 monotherapy was well tolerated and exhibited durable and clinically meaningful efficacy in recurrent H3 K27M-mutant DMG.
Trial registration: ClinicalTrials.gov NCT03416530 NCT03295396 NCT05392374 NCT03134131 NCT02525692.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



References
-
- Vuong HG, Ngo TNM, Le HT, et al. The prognostic significance of HIST1H3B/C and H3F3A K27M mutations in diffuse midline gliomas is influenced by patient age. J Neurooncol. 2022;158:405–412. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

