Efficacy and Safety of Vamorolone Over 48 Weeks in Boys With Duchenne Muscular Dystrophy: A Randomized Controlled Trial
- PMID: 38335499
- PMCID: PMC11067696
- DOI: 10.1212/WNL.0000000000208112
Efficacy and Safety of Vamorolone Over 48 Weeks in Boys With Duchenne Muscular Dystrophy: A Randomized Controlled Trial
Abstract
Background and objectives: Vamorolone is a dissociative agonist of the glucocorticoid receptor that has shown similar efficacy and reduced safety concerns in comparison with prednisone in Duchenne muscular dystrophy (DMD). This study was conducted to determine the efficacy and safety of vamorolone over 48 weeks and to study crossover participants (prednisone to vamorolone; placebo to vamorolone).
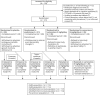
Methods: A randomized, double-blind, placebo-controlled and prednisone-controlled clinical trial of 2 doses of vamorolone was conducted in participants with DMD, in the ages from 4 years to younger than 7 years at baseline. The interventions were 2 mg/kg/d of vamorolone and 6 mg/kg/d of vamorolone for 48 weeks (period 1: 24 weeks + period 2: 24 weeks) and 0.75 mg/kg/d of prednisone and placebo for the first 24 weeks (before crossover). Efficacy was evaluated through gross motor outcomes and safety through adverse events, growth velocity, body mass index (BMI), and bone turnover biomarkers. This analysis focused on period 2.
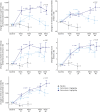
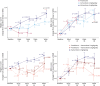
Results: A total of 121 participants with DMD were randomized. Vamorolone at a dose of 6 mg/kg/d showed maintenance of improvement for all motor outcomes to week 48 (e.g., for primary outcome, time to stand from supine [TTSTAND] velocity, week 24 least squares mean [LSM] [SE] 0.052 [0.0130] rises/s vs week 48 LSM [SE] 0.0446 [0.0138]). After 48 weeks, vamorolone at a dose of 2 mg/kg/d showed similar improvements as 6 mg/kg/d for North Star Ambulatory Assessment (NSAA) (vamorolone 6 mg/kg/d-vamorolone 2 mg/kg/d LSM [SE] 0.49 [1.14]; 95% CI -1.80 to 2.78, p = 0.67), but less improvement for other motor outcomes. The placebo to vamorolone 6 mg/kg/d group showed rapid improvements after 20 weeks of treatment approaching benefit seen with 48-week 6 mg/kg/d of vamorolone treatment for TTSTAND, time to run/walk 10 m, and NSAA. There was significant improvement in linear growth after crossover in the prednisone to vamorolone 6 mg/kg/d group, and rapid reversal of prednisone-induced decline in bone turnover biomarkers in both crossover groups. There was an increase in BMI after 24 weeks of treatment that then stabilized for both vamorolone groups.
Discussion: Improvements of motor outcomes seen with 6 mg/kg/d of vamorolone at 24 weeks of treatment were maintained to 48 weeks of treatment. Vamorolone at a dose of 6 mg/kg/d showed better maintenance of effect compared with vamorolone at a dose of 2 mg/kg/d for most (3/5) motor outcomes. Bone morbidities of prednisone (stunting of growth and declines in serum bone biomarkers) were reversed when treatment transitioned to vamorolone.
Trial registration information: ClinicalTrials.gov Identifier: NCT03439670.
Classification of evidence: This study provides Class I evidence that for boys with DMD, the efficacy of vamorolone at a dose of 6 mg/kg/d was maintained over 48 weeks.
Conflict of interest statement
U.J. Dang receives personal fees from ReveraGen Biopharma and grants from Foundation to Eradicate Duchenne. J.M. Damsker receives salary support and stock and stock options from ReveraGen BioPharma and support from grants from the NIH and European Horizons and holds patents related to vamorolone. M. Guglieri receives clinical trial and grant support from ReveraGen BioPharma and grants from the European Commission and the NIH. P.R. Clemens receives grants from the NIH and ReveraGen BioPharma. S.J. Perlman was a site principal investigator of the clinical investigation sponsored by ReveraGen. E.C. Smith was a site principal investigator of the clinical investigation sponsored by ReveraGen. I. Horrocks was a site principal investigator of the clinical investigation sponsored by ReveraGen. R.S. Finkel was a site principal investigator of the clinical investigation sponsored by ReveraGen. J.K. Mah was a site principal investigator of the clinical investigation sponsored by ReveraGen. N. Deconinck was a site principal investigator of the clinical investigation sponsored by ReveraGen. N. Goemans was a site principal investigator of the clinical investigation sponsored by ReveraGen. J. Haberlova was a site principal investigator of the clinical investigation sponsored by ReveraGen. V. Straub was a site principal investigator of the clinical investigation sponsored by ReveraGen. L.J. Mengle-Gaw reported receiving personal fees from ReveraGen BioPharma. B.D. Schwartz reported receiving personal fees from ReveraGen Biopharma. A.D. Harper was a site principal investigator of the clinical investigation sponsored by ReveraGen. P.B. Shieh was a site principal investigator of the clinical investigation sponsored by ReveraGen. L. De Waele was a site principal investigator of the clinical investigation sponsored by ReveraGen. D. Castro was a site principal investigator of the clinical investigation sponsored by ReveraGen. M.L. Yang was a site principal investigator of the clinical investigation sponsored by ReveraGen. M. Ryan was a site principal investigator of the clinical investigation sponsored by ReveraGen. C.M. McDonald was a site principal investigator of the clinical investigation sponsored by ReveraGen. M. Tulinius was a site principal investigator of the clinical investigation sponsored by ReveraGen. R. Webster was a site principal investigator of the clinical investigation sponsored by ReveraGen. H.J. McMillan was a site principal investigator of the clinical investigation sponsored by ReveraGen. N.L. Kuntz was a site principal investigator of the clinical investigation sponsored by ReveraGen. V.K. Rao was a site principal investigator of the clinical investigation sponsored by ReveraGen. G. Baranello was a site principal investigator of the clinical investigation sponsored by ReveraGen. S. Spinty was a site principal investigator of the clinical investigation sponsored by ReveraGen. A.M. Childs was a site principal investigator of the clinical investigation sponsored by ReveraGen. A.M. Sbrocchi was a site principal investigator of the clinical investigation sponsored by ReveraGen. K.A. Selby was a site principal investigator of the clinical investigation sponsored by ReveraGen. M. Monduy was a site principal investigator of the clinical investigation sponsored by ReveraGen. Y. Nevo was a site principal investigator of the clinical investigation sponsored by ReveraGen. J.J. Vilchez-Padilla was a site principal investigator of the clinical investigation sponsored by ReveraGen. A. Nascimento-Osorio was a site principal investigator of the clinical investigation sponsored by ReveraGen. E.H. Niks was a site principal investigator of the clinical investigation sponsored by ReveraGen. I.J.M. de Groot was a site principal investigator of the clinical investigation sponsored by ReveraGen. M. Katsalouli was a site principal investigator of the clinical investigation sponsored by ReveraGen. J. van den Anker received salary support and stock options from ReveraGen. L.M. Ward received contract support from ReveraGen and a grant from Foundation to Eradicate Duchenne. M. Leinonen received salary support from Santhera Pharmaceuticals. A. D'alessandro received contract support from ReveraGen. E.P. Hoffman received salary support and stock from ReveraGen BioPharma and is a PI on grants from the NIH and Food and Drug Administration. Go to
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
