Development of a human liver microphysiological coculture system for higher throughput chemical safety assessment
- PMID: 38335931
- PMCID: PMC11131024
- DOI: 10.1093/toxsci/kfae018
Development of a human liver microphysiological coculture system for higher throughput chemical safety assessment
Abstract
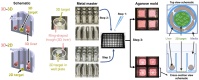
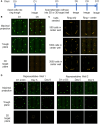
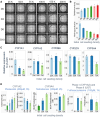
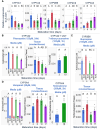
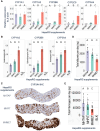
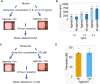
Chemicals in the systemic circulation can undergo hepatic xenobiotic metabolism, generate metabolites, and exhibit altered toxicity compared with their parent compounds. This article describes a 2-chamber liver-organ coculture model in a higher-throughput 96-well format for the determination of toxicity on target tissues in the presence of physiologically relevant human liver metabolism. This 2-chamber system is a hydrogel formed within each well consisting of a central well (target tissue) and an outer ring-shaped trough (human liver tissue). The target tissue chamber can be configured to accommodate a three-dimensional (3D) spheroid-shaped microtissue, or a 2-dimensional (2D) cell monolayer. Culture medium and compounds freely diffuse between the 2 chambers. Human-differentiated HepaRG liver cells are used to form the 3D human liver microtissues, which displayed robust protein expression of liver biomarkers (albumin, asialoglycoprotein receptor, Phase I cytochrome P450 [CYP3A4] enzyme, multidrug resistance-associated protein 2 transporter, and glycogen), and exhibited Phase I/II enzyme activities over the course of 17 days. Histological and ultrastructural analyses confirmed that the HepaRG microtissues presented a differentiated hepatocyte phenotype, including abundant mitochondria, endoplasmic reticulum, and bile canaliculi. Liver microtissue zonation characteristics could be easily modulated by maturation in different media supplements. Furthermore, our proof-of-concept study demonstrated the efficacy of this coculture model in evaluating testosterone-mediated androgen receptor responses in the presence of human liver metabolism. This liver-organ coculture system provides a practical, higher-throughput testing platform for metabolism-dependent bioactivity assessment of drugs/chemicals to better recapitulate the biological effects and potential toxicity of human exposures.
Keywords: in vitro testing; 3D coculture; HepaRG; animal alternatives; liver metabolism; toxicity testing.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




Similar articles
-
High-Throughput Assessment of Metabolism-Mediated Neurotoxicity by Co-Culture of Neurospheres and Liver Spheroids.Curr Protoc. 2024 Oct;4(10):e70023. doi: 10.1002/cpz1.70023. Curr Protoc. 2024. PMID: 39373184
-
From the Cover: Three-Dimensional (3D) HepaRG Spheroid Model With Physiologically Relevant Xenobiotic Metabolism Competence and Hepatocyte Functionality for Liver Toxicity Screening.Toxicol Sci. 2017 Sep 1;159(1):124-136. doi: 10.1093/toxsci/kfx122. Toxicol Sci. 2017. PMID: 28633424 Free PMC article.
-
HepaRG microencapsulated spheroids in DMSO-free culture: novel culturing approaches for enhanced xenobiotic and biosynthetic metabolism.Arch Toxicol. 2015 Aug;89(8):1347-58. doi: 10.1007/s00204-014-1320-9. Epub 2014 Aug 9. Arch Toxicol. 2015. PMID: 25107451
-
The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics.Chem Biol Interact. 2007 May 20;168(1):66-73. doi: 10.1016/j.cbi.2006.12.003. Epub 2006 Dec 16. Chem Biol Interact. 2007. PMID: 17241619 Review.
-
Advanced In Vitro HepaRG Culture Systems for Xenobiotic Metabolism and Toxicity Characterization.Eur J Drug Metab Pharmacokinet. 2019 Aug;44(4):437-458. doi: 10.1007/s13318-018-0533-3. Eur J Drug Metab Pharmacokinet. 2019. PMID: 30535757 Review.
Cited by
-
Human quad liver-on-chip system as a tool toward bridging the gap between animals and humans regarding toxicology and pharmacology of a cannabidiol-rich cannabis extract.Drug Chem Toxicol. 2025 May;48(3):578-585. doi: 10.1080/01480545.2024.2388292. Epub 2024 Aug 19. Drug Chem Toxicol. 2025. PMID: 39155655
-
Spheroid-Hydrogel-Integrated Biomimetic System: A New Frontier in Advanced Three-Dimensional Cell Culture Technology.Cells Tissues Organs. 2025;214(2):128-147. doi: 10.1159/000541416. Epub 2024 Sep 12. Cells Tissues Organs. 2025. PMID: 39265553 Free PMC article. Review.
References
-
- Bimboes D., Greim H. (1976). Human lymphocytes as target cells in a metabolizing test system in vitro for detecting potential mutagens. Mutat. Res. 35, 155–160. - PubMed
-
- Bull S., Langezaal I., Clothier R., Coecke S. (2001). A genetically engineered cell-based system for detecting metabolism-mediated toxicity. Altern. Lab. Anim. 29, 703–716. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

