Intracellular neural control of an active feeding structure in Aplysia using a carbon fiber electrode array
- PMID: 38336092
- PMCID: PMC11136531
- DOI: 10.1016/j.jneumeth.2024.110077
Intracellular neural control of an active feeding structure in Aplysia using a carbon fiber electrode array
Abstract
Background: To study neural control of behavior, intracellular recording and stimulation of many neurons in freely moving animals would be ideal. However, current technologies limit the number of neurons that can be monitored and manipulated. A new technology has become available for intracellular recording and stimulation which we demonstrate in the tractable nervous system of Aplysia.
New method: Carbon fiber electrode arrays (whose tips are coated with platinum-iridium) were used with an in vitro feeding preparation to intracellularly record from and to control the activity of multiple neurons during feeding movements.
Results: In an in vitro feeding preparation, the carbon fiber electrode arrays recorded action potentials and subthreshold synaptic potentials during feeding movements. Depolarizing or hyperpolarizing currents activated or inhibited identified neurons (respectively), manipulating the movements of the feeding apparatus.
Comparison with existing method(s): Standard glass microelectrodes that are commonly used for intracellular recording are stiff, liable to break in response to movement, and require many micromanipulators to be precisely positioned. In contrast, carbon fiber arrays are less sensitive to movement, but are capable of multiple channels of intracellular recording and stimulation.
Conclusions: Carbon fiber arrays are a novel technology for intracellular recording that can be used in moving preparations. They can record both action potentials and synaptic activity in multiple neurons and can be used to stimulate multiple neurons in complex patterns.
Keywords: Aplysia; Carbon fiber electrodes; Intracellular recording; Intracellular stimulation.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interest.
Figures

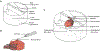
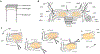



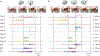

References
-
- Adam Y, Kim JJ, Lou S, Zhao Y, Xie ME, Brinks D, Wu H, Mostajo-Radji MA, Kheifets S, Parot V, Chettih S, Williams KJ, Gmeiner B, Farhi SL, Madisen L, Buchanan EK, Kinsella I, Zhou D, Paninski L, Harvey CD, Zeng H, Arlotta P, Campbell RE, Cohen AE, 2019. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 569, 413–417. 10.1038/s41586-019-1166-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

