Intracerebral haemorrhage in patients taking different types of oral anticoagulants: a pooled individual patient data analysis from two national stroke registries
- PMID: 38336370
- PMCID: PMC11791643
- DOI: 10.1136/svn-2023-002813
Intracerebral haemorrhage in patients taking different types of oral anticoagulants: a pooled individual patient data analysis from two national stroke registries
Abstract
Background: We investigated outcomes in patients with intracerebral haemorrhage (ICH) according to prior anticoagulation treatment with Vitamin K antagonists (VKAs), direct oral anticoagulants (DOACs) or no anticoagulation.
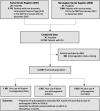
Methods: This is an individual patient data study combining two prospective national stroke registries from Switzerland and Norway (2013-2019). We included all consecutive patients with ICH from both registries. The main outcomes were favourable functional outcome (modified Rankin Scale 0-2) and mortality at 3 months.
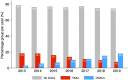
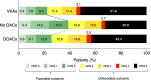
Results: Among 11 349 patients with ICH (mean age 73.6 years; 47.6% women), 1491 (13.1%) were taking VKAs and 1205 (10.6%) DOACs (95.2% factor Xa inhibitors). The median percentage of patients on prior anticoagulation was 23.7 (IQR 22.6-25.1) with VKAs decreasing (from 18.3% to 7.6%) and DOACs increasing (from 3.0% to 18.0%) over time. Prior VKA therapy (n=209 (22.3%); adjusted ORs (aOR), 0.64; 95% CI, 0.49 to 0.84) and prior DOAC therapy (n=184 (25.7%); aOR, 0.64; 95% CI, 0.47 to 0.87) were independently associated with lower odds of favourable outcome compared with patients without anticoagulation (n=2037 (38.8%)). Prior VKA therapy (n=720 (49.4%); aOR, 1.71; 95% CI, 1.41 to 2.08) and prior DOAC therapy (n=460 (39.7%); aOR, 1.28; 95% CI, 1.02 to 1.60) were independently associated with higher odds of mortality compared with patients without anticoagulation (n=2512 (30.2%)).
Conclusions: The spectrum of anticoagulation-associated ICH changed over time. Compared with patients without prior anticoagulation, prior VKA treatment and prior DOAC treatment were independently associated with lower odds of favourable outcome and higher odds of mortality at 3 months. Specific reversal agents unavailable during the study period might improve outcomes of DOAC-associated ICH in the future.
Keywords: Anticoagulants; Hemorrhage; Stroke.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MBo: personal fees from AstraZeneca, a company that produces Andexanet alfa (a specific reversal agent for factor Xa-inhibitor-associated ICH, discussed in this study). SW: consultancy fees from Bayer, a company that produces Rivaroxaban (a DOAC discussed in this study). BV: personal fees from Pfizer AG/Bristol-Myers Squibb SA and Bayer AG, producesr of Apixaban and Rivaroxaban, two drugs discussed in this study. DJS: grants from Alexion/AstraZeneca, producer of andexanet alfa discussed in this study. Personal fees from Bayer, producer of Rivaroxaban, discussed in this study. Consultancy fees from VarmX (producer of VarmX, a compound under development for the treatment of FXaI-associated bleeding). All other authors have nothing to disclose.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
