Melanoma biology and treatment: a review of novel regulated cell death-based approaches
- PMID: 38336727
- PMCID: PMC10858604
- DOI: 10.1186/s12935-024-03220-9
Melanoma biology and treatment: a review of novel regulated cell death-based approaches
Abstract
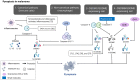
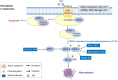
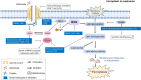
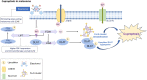
The incidence of melanoma, the most lethal form of skin cancer, has increased due to ultraviolet exposure. The treatment of advanced melanoma, particularly metastatic cases, remains challenging with poor outcomes. Targeted therapies involving BRAF/MEK inhibitors and immunotherapy based on anti-PD1/anti-CTLA4 antibodies have achieved long-term survival rates of approximately 50% for patients with advanced melanoma. However, therapy resistance and inadequate treatment response continue to hinder further breakthroughs in treatments that increase survival rates. This review provides an introduction to the molecular-level pathogenesis of melanoma and offers an overview of current treatment options and their limitations. Cells can die by either accidental or regulated cell death (RCD). RCD is an orderly cell death controlled by a variety of macromolecules to maintain the stability of the internal environment. Since the uncontrolled proliferation of tumor cells requires evasion of RCD programs, inducing the RCD of melanoma cells may be a treatment strategy. This review summarizes studies on various types of nonapoptotic RCDs, such as autophagy-dependent cell death, necroptosis, ferroptosis, pyroptosis, and the recently discovered cuproptosis, in the context of melanoma. The relationships between these RCDs and melanoma are examined, and the interplay between these RCDs and immunotherapy or targeted therapy in patients with melanoma is discussed. Given the findings demonstrating melanoma cell death in response to different stimuli associated with these RCDs, the induction of RCD shows promise as an integral component of treatment strategies for melanoma.
Keywords: Autophagy-dependent cell death; Cuproptosis; Ferroptosis; Immunotherapy; Melanoma; Necroptosis; Pyroptosis; Regulated cell death; Targeted therapy.
© 2024. The Author(s).
Conflict of interest statement
No competing interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

