M2 microglia-derived exosomes promote vascular remodeling in diabetic retinopathy
- PMID: 38336783
- PMCID: PMC10854107
- DOI: 10.1186/s12951-024-02330-w
M2 microglia-derived exosomes promote vascular remodeling in diabetic retinopathy
Abstract
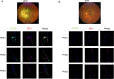
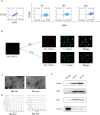
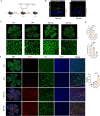
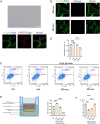
Diabetic retinopathy (DR) is a vision-threatening diabetic complication that is characterized by microvasculature impairment and immune dysfunction. The present study demonstrated that M2 microglia intensively participated in retinal microangiopathy in human diabetic proliferative membranes, mice retinas, retinas of mice with oxygen-induced retinopathy (OIR) mice, and retinas of streptozotocin-induced DR mice. Further in vivo and in vitro experiments showed that exosomes derived from M2 polarized microglia (M2-exo) could reduce pericyte apoptosis and promote endothelial cell proliferation, thereby promoting vascular remodeling and reducing vascular leakage from the diabetic retina. These effects were further enhanced by M2-exo that facilitated M2 polarization of retinal microglia. Collectively, the study demonstrated the capability of M2-exo to induce retinal microvascular remodeling, which may provide a new therapeutic strategy for the treatment of DR.
© 2024. The Author(s).
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

