CAR-T cell therapy targeting surface expression of TYRP1 to treat cutaneous and rare melanoma subtypes
- PMID: 38336975
- PMCID: PMC10858182
- DOI: 10.1038/s41467-024-45221-2
CAR-T cell therapy targeting surface expression of TYRP1 to treat cutaneous and rare melanoma subtypes
Abstract
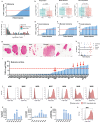
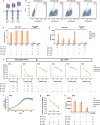
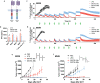
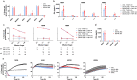
A major limitation to developing chimeric antigen receptor (CAR)-T cell therapies for solid tumors is identifying surface proteins highly expressed in tumors but not in normal tissues. Here, we identify Tyrosinase Related Protein 1 (TYRP1) as a CAR-T cell therapy target to treat patients with cutaneous and rare melanoma subtypes unresponsive to immune checkpoint blockade. TYRP1 is primarily located intracellularly in the melanosomes, with a small fraction being trafficked to the cell surface via vesicular transport. We develop a highly sensitive CAR-T cell therapy that detects surface TYRP1 in tumor cells with high TYRP1 overexpression and presents antitumor activity in vitro and in vivo in murine and patient-derived cutaneous, acral and uveal melanoma models. Furthermore, no systemic or off-tumor severe toxicities are observed in an immunocompetent murine model. The efficacy and safety profile of the TYRP1 CAR-T cell therapy supports the ongoing preparation of a phase I clinical trial.
© 2024. The Author(s).
Conflict of interest statement
A.R. has received honoraria from consulting with Amgen, Bristol-Myers Squibb, Chugai, Dynavax, Genentech, Merck, Nektar, Novartis, Roche, and Sanofi, is or has been a member of the scientific advisory board and holds stock in Advaxis, Arcus Biosciences, Bioncotech Therapeutics, Compugen, CytomX, Five Prime, RAPT, ImaginAb, Isoplexis, Kite-Gilead, Lutris Pharma, Merus, PACT Pharma, Rgenix and Tango Therapeutics. A.R. is a co-founder of PACT Pharma, a member of the Board of Directors, and holds founder stock. Y.Y.C., A.R., and C.P.-S. are founders of, hold equity in, are members of the Scientific Advisory Board, and receive consulting fees from ImmPACT Bio. Y.Y.C. is a member of the scientific advisory board and holds equity in Catamaran Bio, Notch Therapeutics, Pluto Immunotherapeutics, Prime Medicine, Sonoma Biotherapeutics, and Waypoint Bio. She has consulted for Novartis and Gritstone Bio.
UCLA has filed Patent applications on aspects of the described work, entitled “Chimeric Antigen Receptors and Related Methods and Compositions for the Treatment of Cancer” (WO2021046432A1). No potential competing interests were disclosed by the other authors.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

