The Phenotype of Mesenchymal Stromal Cell and Articular Chondrocyte Cocultures on Highly Porous Bilayer Poly-L-Lactic Acid Scaffolds Produced by Thermally Induced Phase Separation and Supplemented with Hydroxyapatite
- PMID: 38337220
- PMCID: PMC10857361
- DOI: 10.3390/polym16030331
The Phenotype of Mesenchymal Stromal Cell and Articular Chondrocyte Cocultures on Highly Porous Bilayer Poly-L-Lactic Acid Scaffolds Produced by Thermally Induced Phase Separation and Supplemented with Hydroxyapatite
Abstract
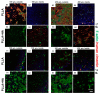
Bilayer scaffolds could provide a suitable topology for osteochondral defect repair mimicking cartilage and subchondral bone architecture. Hence, they could facilitate the chondro- and osteogenic lineage commitment of multipotent mesenchymal stromal cells (MSCs) with hydroxyapatite, the major inorganic component of bone, stimulating osteogenesis. Highly porous poly-L-lactic acid (PLLA) scaffolds with two layers of different pore sizes (100 and 250 µm) and hydroxyapatite (HA) supplementation were established by thermally induced phase separation (TIPS) to study growth and osteogenesis of human (h) MSCs. The topology of the scaffold prepared via TIPS was characterized using scanning electron microscopy (SEM), a microCT scan, pycnometry and gravimetric analysis. HMSCs and porcine articular chondrocytes (pACs) were seeded on the PLLA scaffolds without/with 5% HA for 1 and 7 days, and the cell attachment, survival, morphology, proliferation and gene expression of cartilage- and bone-related markers as well as sulfated glycosaminoglycan (sGAG) synthesis were monitored. All scaffold variants were cytocompatible, and hMSCs survived for the whole culture period. Cross-sections revealed living cells that also colonized inner scaffold areas, producing an extracellular matrix (ECM) containing sGAGs. The gene expression of cartilage and bone markers could be detected. HA represents a cytocompatible supplement in PLLA composite scaffolds intended for osteochondral defects.
Keywords: chondrocytes; chondrogenesis; hydroxyapatite; mesenchymal stromal cells; osteochondral defects; osteogenesis; poly-L-lactic acid; tissue engineering.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Klimek K., Benko A., Vandrovcova M., Travnickova M., Douglas T.E., Tarczynska M., Broz A., Gaweda K., Ginalska G., Bacakova L. Biomimetic biphasic curdlan-based scaffold for osteochondral tissue engineering applications—Characterization and preliminary evaluation of mesenchymal stem cell response in vitro. Biomater. Adv. 2022;135:212724. doi: 10.1016/j.bioadv.2022.212724. - DOI - PubMed
-
- Ma W., Zhou Z., Ismail N., Tocci E., Figoli A., Khayet M., Matsuura T., Cui Z., Tavajohi N. Membrane formation by thermally induced phase separation: Materials, involved parameters, modeling, current efforts and future directions. J. Membr. Sci. 2023;669:121303. doi: 10.1016/j.memsci.2022.121303. - DOI
-
- Pavia F.C., La Carrubba V., Piccarolo S., Brucato V. Polymeric scaffolds prepared via thermally induced phase separation: Tuning of structure and morphology. J. Biomed. Mater. Res. Part A. 2007;86:459–466. - PubMed
LinkOut - more resources
Full Text Sources

