Features of Changes in the Structure and Properties of a Porous Polymer Material with Antibacterial Activity during Biodegradation in an In Vitro Model
- PMID: 38337268
- PMCID: PMC10857194
- DOI: 10.3390/polym16030379
Features of Changes in the Structure and Properties of a Porous Polymer Material with Antibacterial Activity during Biodegradation in an In Vitro Model
Abstract
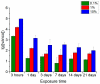
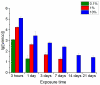
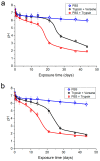
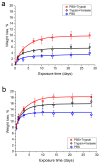
Hybrid porous polymers based on poly-EGDMA and polylactide containing vancomycin, the concentration of which in the polymer varied by two orders of magnitude, were synthesized. The processes of polymer biodegradation and vancomycin release were studied in the following model media: phosphate-buffered saline (PBS), trypsin-Versene solution, and trypsin-PBS solution. The maximum antibiotic release was recorded during the first 3 h of extraction. The duration of antibiotic escape from the polymer samples in trypsin-containing media varied from 3 to 22 days, depending on the antibiotic content of the polymer. Keeping samples of the hybrid polymer in trypsin-containing model media resulted in acidification of the solutions-after 45 days, up to a pH of 1.84 in the trypsin-Versene solution and up to pH 1.65 in the trypsin-PBS solution. Here, the time dependences of the vancomycin release from the polymer into the medium and the decrease in pH of the medium correlated. These data are also consistent with the results of a study of the dynamics of sample weight loss during extraction in the examined model media. However, while the polymer porosity increased from ~53 to ~60% the pore size changed insignificantly, over only 10 μm. The polymer samples were characterized by their antibacterial activity against Staphylococcus aureus, and this activity persisted for up to 21 days during biodegradation of the material, regardless of the medium type used in model. Surface-dependent human cells (dermal fibroblasts) adhere well, spread out, and maintain high viability on samples of the functionalized hybrid polymer, thus demonstrating its biocompatibility in vitro.
Keywords: antibiotic release; cell adhesion; ethylene glycol dimethacrylate; photopolymerization; polylactide; porosity; porous polymer; scaffolds; scanning electron microscopy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Porous Polymer Scaffolds based on Cross-Linked Poly-EGDMA and PLA: Manufacture, Antibiotics Encapsulation, and In Vitro Study.Macromol Biosci. 2021 May;21(5):e2000402. doi: 10.1002/mabi.202000402. Epub 2021 Mar 23. Macromol Biosci. 2021. PMID: 33759338
-
Antibiotic-loaded hydroxyapatite scaffolds fabricated from Nile tilapia bones for orthopaedics.Int J Pharm X. 2023 Feb 8;5:100169. doi: 10.1016/j.ijpx.2023.100169. eCollection 2023 Dec. Int J Pharm X. 2023. PMID: 36861068 Free PMC article.
-
Pore Structure Tuning of Poly-EGDMA Biomedical Material by Varying the O-Quinone Photoinitiator.Polymers (Basel). 2023 Jun 2;15(11):2558. doi: 10.3390/polym15112558. Polymers (Basel). 2023. PMID: 37299356 Free PMC article.
-
Porous devices derived from co-continuous polymer blends as a route for controlled drug release.Biomacromolecules. 2008 Apr;9(4):1131-8. doi: 10.1021/bm7010467. Epub 2008 Mar 21. Biomacromolecules. 2008. PMID: 18355029
-
Hydroxyapatite porous scaffold engineered with biological polymer hybrid coating for antibiotic Vancomycin release.J Mater Sci Mater Med. 2005 Mar;16(3):189-95. doi: 10.1007/s10856-005-6679-y. J Mater Sci Mater Med. 2005. PMID: 15744609
Cited by
-
Specifics of Porous Polymer and Xenogeneic Matrices and of Bone Tissue Regeneration Related to Their Implantation into an Experimental Rabbit Defect.Polymers (Basel). 2024 Apr 20;16(8):1165. doi: 10.3390/polym16081165. Polymers (Basel). 2024. PMID: 38675083 Free PMC article.
References
-
- Kovylin R., Aleynik D.Y., Fedushkin I. Modern Porous Polymer Implants: Synthesis, Properties, and Application. Polym. Sci. Ser. C. 2021;63:29–46. doi: 10.1134/S1811238221010033. - DOI
-
- Chesnokov S.A., Aleynik D.Y., Kovylin R.S., Yudin V.V., Egiazaryan T.A., Egorikhina M.N., Zaslavskaya M.I., Rubtsova Y.P., Gusev S.A., Mlyavykh S.G. Porous Polymer Scaffolds based on Cross-Linked Poly-EGDMA and PLA: Manufacture, Antibiotics Encapsulation, and In Vitro Study. Macromol. Biosci. 2021;21:2000402. doi: 10.1002/mabi.202000402. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

