Status of Polymer Fused Deposition Modeling (FDM)-Based Three-Dimensional Printing (3DP) in the Pharmaceutical Industry
- PMID: 38337275
- PMCID: PMC10857269
- DOI: 10.3390/polym16030386
Status of Polymer Fused Deposition Modeling (FDM)-Based Three-Dimensional Printing (3DP) in the Pharmaceutical Industry
Abstract
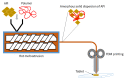
Additive manufacturing (AM) or 3D printing (3DP) is arguably a versatile and more efficient way for the production of solid dosage forms such as tablets. Of the various 3DP technologies currently available, fused deposition modeling (FDM) includes unique characteristics that offer a range of options in the production of various types of tablets. For example, amorphous solid dispersions (ASDs), enteric-coated tablets or poly pills can be produced using an appropriate drug/polymer combination during FDM 3DP. The technology offers the possibility of evolving personalized medicines into cost-effective production schemes at pharmacies and hospital dispensaries. In this review, we highlight key FDM features that may be exploited for the production of tablets and improvement of therapy, with emphasis on gastrointestinal delivery. We also highlight current constraints that must be surmounted to visualize the deployment of this technology in the pharmaceutical and healthcare industries.
Keywords: amorphous solid dispersion; fused deposition modeling; pharmaceutical; polymer; solubility; three-dimensional printing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Fused Deposition Modelling 3D printing and solubility improvement of BCS II and IV active ingredients - A narrative review.J Control Release. 2024 Jan;365:507-520. doi: 10.1016/j.jconrel.2023.11.041. Epub 2023 Dec 2. J Control Release. 2024. PMID: 38036003 Review.
-
The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique: Material considerations-printability-process modulation, with consecutive effects on in vitro release, stability and degradation.Eur J Pharm Sci. 2019 Mar 1;129:110-123. doi: 10.1016/j.ejps.2018.12.019. Epub 2019 Jan 2. Eur J Pharm Sci. 2019. PMID: 30610954
-
An Overview of 3D Printing Technologies for Soft Materials and Potential Opportunities for Lipid-based Drug Delivery Systems.Pharm Res. 2018 Nov 7;36(1):4. doi: 10.1007/s11095-018-2531-1. Pharm Res. 2018. PMID: 30406349 Review.
-
Performance and paroxetine stability in tablets manufactured by fused deposition modelling-based 3D printing.J Pharm Pharmacol. 2022 Jan 5;74(1):67-76. doi: 10.1093/jpp/rgab138. J Pharm Pharmacol. 2022. PMID: 34591102
-
An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing.Eur J Pharm Biopharm. 2016 Nov;108:111-125. doi: 10.1016/j.ejpb.2016.08.016. Epub 2016 Sep 2. Eur J Pharm Biopharm. 2016. PMID: 27594210
Cited by
-
Expanding the Manufacturing Approaches for Gastroretentive Drug Delivery Systems with 3D Printing Technology.Pharmaceutics. 2024 Jun 11;16(6):790. doi: 10.3390/pharmaceutics16060790. Pharmaceutics. 2024. PMID: 38931911 Free PMC article. Review.
-
Fabrication of Polypill Pharmaceutical Dosage Forms Using Fused Deposition Modeling 3D Printing: A Systematic Review.Pharmaceutics. 2024 Sep 30;16(10):1285. doi: 10.3390/pharmaceutics16101285. Pharmaceutics. 2024. PMID: 39458614 Free PMC article. Review.
-
Mechanistic Insights into Amorphous Solid Dispersions: Bridging Theory and Practice in Drug Delivery.Pharm Res. 2025 Jan;42(1):1-23. doi: 10.1007/s11095-024-03808-w. Epub 2025 Jan 23. Pharm Res. 2025. PMID: 39849216 Review.
-
Pharmacokinetic assessment and level-A IVIVC establishment of rifampicin-loaded 3D printed tablets using SLS 3D printing.Ther Deliv. 2025 Jun;16(6):535-544. doi: 10.1080/20415990.2025.2484169. Epub 2025 Mar 30. Ther Deliv. 2025. PMID: 40159706
-
Sex-specific formulations of doxazosin mesylate via direct powder extrusion 3D printing.Drug Deliv Transl Res. 2025 Sep;15(9):3338-3350. doi: 10.1007/s13346-025-01862-4. Epub 2025 Apr 22. Drug Deliv Transl Res. 2025. PMID: 40263229 Free PMC article.
References
-
- Mwema F.M., Akinlabi E.T. Fused Deposition Modeling. Springer Nature; Berlin/Heidelberg, Germany: 2020. Basics of Fused Deposition Modelling (FDM) pp. 1–15. - DOI
-
- Gibson I., Rosen D., Stucker B. Additive Manufacturing Technologies. Springer; New York, NY, USA: 2015. - DOI
-
- Yan Q., Dong H., Su J., Han J., Song B., Wei Q., Shi Y. A Review of 3D Printing Technology for Medical Applications. Engineering. 2018;4:729–742. doi: 10.1016/j.eng.2018.07.021. - DOI
-
- Shahrubudin N., Lee T.C., Ramlan R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019;35:1286–1296. doi: 10.1016/j.promfg.2019.06.089. - DOI
Publication types
LinkOut - more resources
Full Text Sources

