Never Too Late: Safety and Efficacy of Deep TMS for Late-Life Depression
- PMID: 38337509
- PMCID: PMC10856385
- DOI: 10.3390/jcm13030816
Never Too Late: Safety and Efficacy of Deep TMS for Late-Life Depression
Abstract
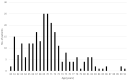
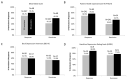
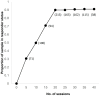
Repetitive transcranial magnetic stimulation (rTMS) is an effective and well-established treatment for major depressive disorder (MDD). Deep TMS utilizes specially designed H-Coils to stimulate the deep and broad cerebral regions associated with the reward system. The improved depth penetration of Deep TMS may be particularly important in late-life patients who often experience brain atrophy. The aim of this phase IV open-label study was to evaluate the safety and efficacy of Deep TMS in patients with late-life MDD. Data were collected from 247 patients with MDD aged 60-91 at 16 sites who had received at least 20 Deep TMS sessions for MDD. The outcome measures included self-assessment questionnaires (Patient Health Questionnaire-9 (PHQ-9), Beck Depression Inventory-II (BDI-II)) and clinician-based scales (21-item Hamilton Depression Rating Scale (HDRS-21)). Following 30 sessions of Deep TMS, there was a 79.4% response and 60.3% remission rate on the most rated scale. The outcomes on the PHQ-9 were similar (76.6% response and 54.7% remission rate). The highest remission and response rates were observed with the HDRS physician-rated scale after 30 sessions (89% response and a 78% remission rate). After 20 sessions, there was a 73% response and 73% remission rate on the HDRS. Consistent with prior studies, the median onset of response was 14 sessions (20 days). The median onset of remission was 15 sessions (23 days). The treatment was well tolerated, with no reported serious adverse events. These high response and remission rates in patients with treatment-resistant late-life depression suggest that Deep TMS is a safe, well-tolerated and effective treatment for this expanded age range of older adults.
Keywords: Deep TMS; H-coil; late-life depression; major depressive disorder; phase IV study; rTMS.
Conflict of interest statement
Declaration of Interest: Yiftach Roth and Colleen A. Hanlon are employees of and have a financial interest in BrainsWay, a TMS device manufacturer. Aron Tendler is a consultant for and has a financial interest in BrainsWay, as well as a commercial TMS center. Steven A. Harvey and Faisal Munasifi have a financial interest in a commercial TMS center. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- United Nations, Department of Economic and Social Affairs, Population Division . World Population Ageing, 2019 Highlights. United Nations; New York, NY, USA: 2020.
-
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR *D report. Am. J. Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

