Topical Treatment Is Effective and Safe for Acute Ankle Sprains: The Multi-Center Double-Blind Randomized Placebo-Controlled TRAUMED Trial
- PMID: 38337536
- PMCID: PMC10856131
- DOI: 10.3390/jcm13030841
Topical Treatment Is Effective and Safe for Acute Ankle Sprains: The Multi-Center Double-Blind Randomized Placebo-Controlled TRAUMED Trial
Abstract
Background: Topical NSAIDs are widely used to treat ankle sprains. Traumed (Tr14) gel is a multicomponent formulation, demonstrating inflammation-resolution properties.
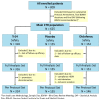
Methods: This multicenter, double-blind trial investigated the efficacy and safety of Tr14 gel versus placebo gel and non-inferiority versus 1% diclofenac gel, applied 3×/day for 7 days after acute lateral ankle sprain (EudraCT Number: 2016-004792-50). The primary outcome was AUC for pain on passive movement, assessed by VAS from baseline to Days 4 and 7.
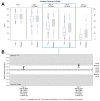
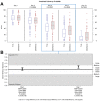
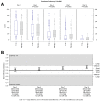
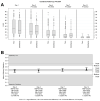
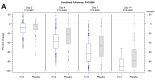
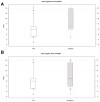
Results: The trial population included 625 patients aged 18 to 78 years. The AUC scores were 187.88 and 200.75 on Day 4 (p = 0.02) and 294.14 and 353.42 on Day 7 (p < 0.001) for Tr14 and placebo, respectively. For Tr14 compared to diclofenac, the AUC scores were 187.50 and 197.19 on Day 4 (p = 0.3804) and 293.85 and 327.93 on Day 7 (p = 0.0017), respectively. On the FAAM-ADL subscale, Tr14 was superior to placebo and non-inferior to diclofenac at all time points. Time to 50% pain improvement was lowest for Tr14 (6.0 days), compared to placebo (7.1 days) and diclofenac (7.0 days). Adverse events were uncommon and minor.
Conclusions: Tr14 gel is effective and safe in acute ankle sprains, compared to placebo gel and diclofenac gel, and has faster pain resolution.
Trial registration: The trial was registered in clinicaltrialsregister.eu, EudraCT number 2016-004792-50 on 07.06.2017.
Keywords: TRAUMED; Tr14; ankle sprain; anti-inflammatory agents; diclofenac; injury; musculoskeletal pain; traumeel; treatment.
Conflict of interest statement
Ludger Gerdesmeyer, Christian Schneider, Britt Wildemann, and Gino Kerkhoffs received consultancy fees to participate in Advisory Board meetings during the conception, conduct, and analysis of this clinical trial. Johannes Vester is employed by idv Data Analysis and Study Planning, the CRO contracted by Heel for statistical analyses. Christine Frank, Myron Schultz, and Alta Smit are employees of Heel GmbH. Bernd Seilheimer was employed at Heel GmbH when the trial was designed and conducted.
Figures

Similar articles
-
Tr14 gel for the treatment of acute ankle sprains: a plain language summary of the TRAUMED trial.J Comp Eff Res. 2025 Jul;14(7):e250018. doi: 10.57264/cer-2025-0018. Epub 2025 Jun 5. J Comp Eff Res. 2025. PMID: 40468690 Free PMC article. Clinical Trial.
-
Diclofenac epolamine plus heparin plaster versus diclofenac epolamine plaster in mild to moderate ankle sprain: a randomized, double-blind, parallel-group, placebo-controlled, multicentre, phase III trial.Clin Drug Investig. 2011;31(1):15-26. doi: 10.2165/11585890-000000000-00000. Clin Drug Investig. 2011. PMID: 20923251 Clinical Trial.
-
Traumeel vs. diclofenac for reducing pain and improving ankle mobility after acute ankle sprain: a multicentre, randomised, blinded, controlled and non-inferiority trial.Int J Clin Pract. 2013 Oct;67(10):979-89. doi: 10.1111/ijcp.12219. Epub 2013 Jul 25. Int J Clin Pract. 2013. PMID: 23889885 Free PMC article. Clinical Trial.
-
Pooled analysis of clinical trial data evaluating the safety and effectiveness of diclofenac epolamine topical patch 1.3% for the treatment of acute ankle sprain.Open Access J Sports Med. 2011 Jul 14;2:75-84. doi: 10.2147/OAJSM.S17048. Open Access J Sports Med. 2011. PMID: 24198574 Free PMC article. Review.
-
Systematic review of topical diclofenac for the treatment of acute and chronic musculoskeletal pain.Curr Med Res Opin. 2020 Apr;36(4):637-650. doi: 10.1080/03007995.2020.1716703. Epub 2020 Feb 3. Curr Med Res Opin. 2020. PMID: 31944135
References
-
- Gribble P.A., Bleakley C.M., Caulfield B.M., Docherty C.L., Fourchet F., Fong D.T.-P., Hertel J., Hiller C.E., Kaminski T.W., McKeon P.O., et al. Evidence review for the 2016 International Ankle Consortium consensus statement on the prevalence, impact and long-term consequences of lateral ankle sprains. Br. J. Sports Med. 2016;50:1496–1505. doi: 10.1136/bjsports-2016-096189. - DOI - PubMed
LinkOut - more resources
Full Text Sources

