Approach to Acute Myeloid Leukemia with Increased Eosinophils and Basophils
- PMID: 38337573
- PMCID: PMC10856720
- DOI: 10.3390/jcm13030876
Approach to Acute Myeloid Leukemia with Increased Eosinophils and Basophils
Abstract
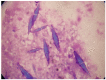
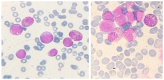
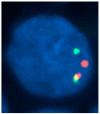
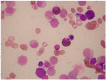
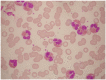
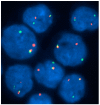
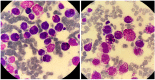
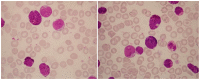
There is remarkable morphologic and genetic heterogeneity in acute myeloid leukemia (AML). In a small percentage of cases of AML, increased eosinophils and/or basophils are present in the bone marrow and sometimes in the peripheral blood. This is often a puzzling diagnostic situation but also an important finding that requires special investigation. Unique chromosomal rearrangements have been correlated with an increased number of eosinophils and basophils in AML. The identification of the underlying genetic lesion that promotes eosinophilia and basophilia can dramatically change both the prognosis and the treatment of the patient. Thus, clinicians must be vigilant in searching for the cause of eosinophilia and basophilia in patients with AML, since the different causes may lead to different treatments and survival outcomes. In this article, we examine the significance of increased eosinophils and/or basophils in the context of AML, provide guidance that simplifies the differential diagnosis, and give prognostic and therapeutic information about specific subtypes of AML associated with eosinophilia and/or basophilia. Evidence supporting personalized (molecularly targeted) therapy for these patients is also presented.
Keywords: AML; CBF; PDGFRA; PDGFRB; acute myeloid leukemia; basophil; basophilia; eosinophil; eosinophilia; morphology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Hoffman R., Benz E., Silberstein L., Heslop H., Weitz J., Anastasi J., Salama M. Hematology: Basic Principles and Practice. Elsevier; Philadelphia, PA, USA: 2018. pp. 330–331. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

