The Impact of Extracorporeal Magnetic Stimulation as Addition to Mirabegron in Overactive Bladder Treatment in Women: A Single-Centre Randomized Sham-Controlled Study
- PMID: 38337609
- PMCID: PMC10856599
- DOI: 10.3390/jcm13030916
The Impact of Extracorporeal Magnetic Stimulation as Addition to Mirabegron in Overactive Bladder Treatment in Women: A Single-Centre Randomized Sham-Controlled Study
Abstract
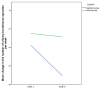
(1) Background: The purpose of our prospective, single-blinded, randomized, sham-controlled study was to investigate the effect of the additional extracorporeal magnetic stimulation (ExMI) to pharmacological treatment in overactive bladder syndrome (OAB) in women. (2) Methods: We recruited 56 women with OAB, who were allocated into two study groups: the active group received mirabegron 50 mg daily and a total of 16 sessions of ExMI in 8 weeks, whereas the sham group received mirabegron 50 mg daily and sham stimulation following the same treatment protocol. Treatment success was evaluated after 4 and 8 weeks. (3) Results: Both groups experienced significant reduction in daytime urinary frequency, nocturia, and number of weekly incontinence episodes after 8 weeks. There were no statistically significant differences in end-point daytime urinary frequency and nocturia between groups. However, the overall average reduction rate in weekly number of incontinence episodes was 43.7% in treatment group and 24.2% in the control group. The number of urinary incontinence episodes in the treatment and control group was reduced for 3.8 ± 11.8 vs. 2.5 ± 4.3 episodes at week 4 and additional 3.3 ± 6 vs. 0.4 ± 3.2 episodes at week 8, respectively (p = 0.013). Moreover, IIQ-7 score showed a significantly greater score reduction and patients' evaluated improvement of symptoms was higher in the active group. (4) Conclusions: The addition of ExMI to mirabegron in OAB treatment further improves the weekly incontinence episode reduction rate and also leads to grater improvement in symptoms.
Keywords: magnetic stimulation; mirabegron; overactive bladder; patient satisfaction; urge urinary incontinence; urgency.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Haylen B.T., Maher C.F., Barber M.D., Camargo S., Dandolu V., Digesu A., Goldman H.B., Huser M., Milani A.L., Moran P.A., et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Organ Prolapse (POP) Int. Urogynecol. J. 2016;27:165–194. doi: 10.1007/s00192-015-2932-1. - DOI - PubMed
-
- Irwin D.E., Milsom I., Hunskaar S., Reilly K., Kopp Z., Herschorn S., Coyne K., Kelleher C., Hampel C., Artibani W., et al. Population-Based Survey of Urinary Incontinence, Overactive Bladder, and Other Lower Urinary Tract Symptoms in Five Countries: Results of the EPIC Study. Eur. Urol. 2006;50:1306–1315. doi: 10.1016/j.eururo.2006.09.019. - DOI - PubMed
-
- Coyne K.S., Wein A.J., Tubaro A., Sexton C.C., Thompson C.L., Kopp Z.S., Aiyer L.P. The Burden of Lower Urinary Tract Symptoms: Evaluating the Effect of LUTS on Health-Related Quality of Life, Anxiety and Depression: EpiLUTS. BJU Int. 2009;103:4–11. doi: 10.1111/j.1464-410X.2009.08371.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

