Efficacy and Safety of a Probiotic Containing Saccharomyces boulardii CNCM I-745 in the Treatment of Small Intestinal Bacterial Overgrowth in Decompensated Cirrhosis: Randomized, Placebo-Controlled Study
- PMID: 38337613
- PMCID: PMC10856456
- DOI: 10.3390/jcm13030919
Efficacy and Safety of a Probiotic Containing Saccharomyces boulardii CNCM I-745 in the Treatment of Small Intestinal Bacterial Overgrowth in Decompensated Cirrhosis: Randomized, Placebo-Controlled Study
Abstract
(1) Background: The aim was to evaluate the effectiveness of the probiotic containing Saccharomyces boulardii in the treatment of small intestinal bacterial overgrowth (SIBO) in patients with decompensated cirrhosis. (2) Methods: This was a blinded, randomized, placebo-controlled study. (3) Results: After 3 months of treatment, SIBO was absent in 80.0% of patients in the probiotic group and in 23.1% of patients in the placebo group (p = 0.002). The patients with eliminated SIBO had decreased frequency of ascites and hepatic encephalopathy, the increased platelets and albumin levels, the decreased blood levels of total bilirubin, biomarkers of bacterial translocation (lipopolysaccharide [LPS]) and systemic inflammation (C-reactive protein), and positive changes in markers of hyperdynamic circulation compared with the state at inclusion. There were no significant changes in the claudin 3 level (the intestinal barrier biomarker) in these patients. No significant changes were observed in the group of patients with persistent SIBO. The serum level of nitrate (endothelial dysfunction biomarker) was lower in patients with eradicated SIBO than in patients with persistent SIBO. One (5.3%) patient with eradicated SIBO and six (42.9%) patients with persistent SIBO died within the first year of follow-up (p = 0.007). (4) Conclusions: SIBO eradication was an independent predictor of a favorable prognosis during the first year of follow-up.
Keywords: endotoxemia; gut–heart axis; gut–liver axis; hemodynamics; leaky gut; liver; microbiota; probiotics; vasodilatation.
Conflict of interest statement
The sponsor did not participate in the development of the design and did not influence the course of the study, the processing of the results and the decision to publish.
Figures
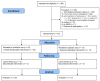

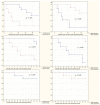
References
-
- GBD 2017 Cirrhosis Collaborators The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. - DOI - PMC - PubMed
-
- Ma C., Qian A.S., Nguyen N.H., Stukalin I., Congly S.E., Shaheen A.A., Swain M.G., Teriaky A., Asrani S.K., Singh S. Trends in the economic burden of chronic liver diseases and cirrhosis in the United States: 1996–2016. Am. J. Gastroenterol. 2021;116:2060–2067. doi: 10.14309/ajg.0000000000001292. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

