Kinetics of Cellular Cobalamin Uptake and Conversion: Comparison of Aquo/Hydroxocobalamin to Cyanocobalamin
- PMID: 38337663
- PMCID: PMC10857013
- DOI: 10.3390/nu16030378
Kinetics of Cellular Cobalamin Uptake and Conversion: Comparison of Aquo/Hydroxocobalamin to Cyanocobalamin
Abstract
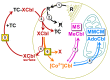
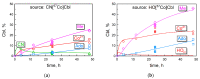
Cyanocobalamin (CNCbl) and aquo/hydroxocobalamin (HOCbl) are the forms of vitamin B12 that are most commonly used for supplementation. They are both converted to methylcobalamin (MeCbl) and 5'-deoxyadenosylcobalamin (AdoCbl), which metabolize homocysteine and methylmalonic acid, respectively. Here, we compare the kinetics of uptake and the intracellular transformations of radiolabeled CNCbl vs. HOCbl in HeLa cells. More HOCbl was accumulated over 4-48 h, but further extrapolation indicated similar uptake (>90%) for both vitamin forms. The initially synthesized coenzyme was MeCbl, which noticeably exceeded AdoCbl during 48 h. Yet, the synthesis of AdoCbl accelerated, and the predicted final levels of Cbls were MeCbl ≈ AdoCbl ≈ 40% and HOCbl ≈ 20%. The designed kinetic model revealed the same patterns of the uptake and turnover for CNCbl and HOCbl, apart from two steps. First, the "activating" intracellular processing of the internalized HOCbl was six-fold faster. Second, the detachment rates from the cell surface (when the "excessive" Cbl-molecules were refluxed into the external medium) related as 4:1 for CNCbl vs. HOCbl. This gave a two-fold faster cellular accumulation and processing of HOCbl vs. CNCbl. In medical terms, our data suggest (i) an earlier response to the treatment of Cbl-deficiency with HOCbl, and (ii) the manifestation of a successful treatment initially as a decrease in homocysteine.
Keywords: HeLa cells; cobalamin; kinetics; uptake; vitamin B12.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency.Mol Nutr Food Res. 2015 Jul;59(7):1364-72. doi: 10.1002/mnfr.201500019. Epub 2015 May 12. Mol Nutr Food Res. 2015. PMID: 25820384 Free PMC article. Review.
-
Low methylcobalamin in liver tissues is an artifact as shown by a revised extraction procedure.Biochim Biophys Acta Gen Subj. 2023 Apr;1867(4):130315. doi: 10.1016/j.bbagen.2023.130315. Epub 2023 Feb 4. Biochim Biophys Acta Gen Subj. 2023. PMID: 36739999
-
Intracellular processing of vitamin B12 by MMACHC (CblC).Vitam Horm. 2022;119:275-298. doi: 10.1016/bs.vh.2022.02.001. Epub 2022 Mar 15. Vitam Horm. 2022. PMID: 35337623 Review.
-
Liquid chromatographic mass spectrometric (LC/MS/MS) determination of plasma hydroxocobalamin and cyanocobalamin concentrations after hydroxocobalamin antidote treatment for cyanide poisoning.J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Sep 15;905:10-6. doi: 10.1016/j.jchromb.2012.07.012. Epub 2012 Aug 9. J Chromatogr B Analyt Technol Biomed Life Sci. 2012. PMID: 22939270
-
Differences in the Formation of Reactive Oxygen Species and Their Cytotoxicity between Thiols Combined with Aqua- and Cyanocobalamins.Int J Mol Sci. 2022 Sep 20;23(19):11032. doi: 10.3390/ijms231911032. Int J Mol Sci. 2022. PMID: 36232333 Free PMC article.
Cited by
-
Optimization of Cyanocobalamin (Vitamin B12) Sorption onto Mesoporous Superparamagnetic Iron Oxide Nanoparticles.Molecules. 2024 May 1;29(9):2094. doi: 10.3390/molecules29092094. Molecules. 2024. PMID: 38731585 Free PMC article.
-
Novel Method for the Synthesis of Hydroxycobalamin[c-lactam] and Its Impact on Melanoma Cells In Vitro.Int J Mol Sci. 2025 Feb 12;26(4):1540. doi: 10.3390/ijms26041540. Int J Mol Sci. 2025. PMID: 40004003 Free PMC article.
-
From Nutrient to Nanocarrier: The Multifaceted Role of Vitamin B12 in Drug Delivery.Int J Mol Sci. 2025 May 26;26(11):5119. doi: 10.3390/ijms26115119. Int J Mol Sci. 2025. PMID: 40507930 Free PMC article. Review.
References
-
- Schneider Z., Stroinski A. Comprehensive B12. Chemistry, Biochemistry, Nutrition, Ecology, Medicine. De Gruyter; Berlin, Germany: New York, NY, USA: 1987. - DOI
-
- Sobczyńska-Malefora A., Delvin E., McCaddon A., Ahmadi K.R., Harrington D.J. Vitamin B12 status in health and disease: A critical review. Diagnosis of deficiency and insufficiency—Clinical and laboratory pitfalls. Crit. Rev. Clin. Lab. Sci. 2021;58:399–429. doi: 10.1080/10408363.2021.1885339. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

