Efficacy of Lactiplantibacillus plantarum PBS067, Bifidobacterium animalis subsp. lactis BL050, and Lacticaseibacillus rhamnosus LRH020 in the Amelioration of Vaginal Microbiota in Post-Menopausal Women: A Prospective Observational Clinical Trial
- PMID: 38337685
- PMCID: PMC10857347
- DOI: 10.3390/nu16030402
Efficacy of Lactiplantibacillus plantarum PBS067, Bifidobacterium animalis subsp. lactis BL050, and Lacticaseibacillus rhamnosus LRH020 in the Amelioration of Vaginal Microbiota in Post-Menopausal Women: A Prospective Observational Clinical Trial
Abstract
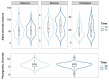
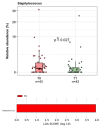
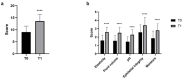
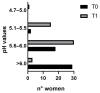
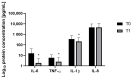
The menopausal transition marks a significant physiological shift in women. Menopause-related symptoms can significantly affect a woman's quality of life and probiotics have emerged as a promising avenue. This study aims to investigate the benefits of probiotics in improving vaginal well-being and microbiota composition in post-menopausal women. A prospective observational clinical trial was carried out enrolling 50 post-menopausal healthy women, aged between 45 and 65 years old, taking a supplement containing Lactiplantibacillus plantarum PBS067, Bifidobacterium animalis subsp. lactis BL050, and Lacticaseibacillus rhamnosus LRH020 (3B CFU/day) for 28 days. Vaginal swabs were collected to evaluate microbiota fluctuation and the inflammatory pattern was recorded. A Vaginal Health Index was provided to evaluate vaginal well-being throughout the trial. Clinical outcomes revealed a decrease in menopausal symptoms. Significant improvements were observed across various parameters: a 50% enhancement in the VHI score (p < 0.0001), alongside substantial reductions in inflammatory cytokine levels. An 87.8% decrease in IL-6, 57.6% in IL-1β, and 40.8% in TNF-α was observed (p < 0.05). Moreover, the probiotic intervention facilitated the restoration of vaginal microbiota, evidenced by an increase in lactobacilli abundance. In conclusion, the combination of these specific probiotic strains, previously clinically tested in childbearing-age women, showed to be effective also for post-menopausal women.
Keywords: genitourinary syndrome of menopause; immunity; lactobacilli; menopause; pro-inflammatory cytokines; probiotics; vaginal atrophy; vaginal microbiota.
Conflict of interest statement
Authors P.M. and D.F.S. were employed by the company Synbalance Srl. Authors E.V., A.M. and A.C. were employed by the company Wellmicro Srl. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Comparetto C., Borruto F. Treatments and Management of Menopausal Symptoms: Current Status and Future Challenges. OBM Geriatr. 2023;7:1–47. doi: 10.21926/obm.geriatr.2303248. - DOI
-
- Harlow S.D., Gass M., Hall J.E., Lobo R., Maki P., Rebar R.W., Sherman S., Sluss P.M., De Villiers T.J. Executive Summary of the Stages of Reproductive Aging Workshop + 10: Addressing the Unfinished Agenda of Staging Reproductive Aging. Menopause. 2012;19:387–395. doi: 10.1097/gme.0b013e31824d8f40. - DOI - PMC - PubMed
-
- Rindner L., Nordeman L., Strömme G., Hange D., Gunnarsson R., Rembeck G. Effect of Group Education and Person-Centered Support in Primary Health Care on Mental Health and Quality of Life in Women Aged 45–60 Years with Symptoms Commonly Associated with Stress: A Randomized Controlled Trial. BMC Womens Health. 2023;23:128. doi: 10.1186/s12905-023-02221-6. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

