The Effect of Alginate Encapsulated Plant-Based Carbohydrate and Protein Supplementation on Recovery and Subsequent Performance in Athletes
- PMID: 38337697
- PMCID: PMC10857232
- DOI: 10.3390/nu16030413
The Effect of Alginate Encapsulated Plant-Based Carbohydrate and Protein Supplementation on Recovery and Subsequent Performance in Athletes
Abstract
The main purpose of this study was to investigate the effect of a novel alginate-encapsulated carbohydrate-protein (CHO-PRO ratio 2:1) supplement (ALG) on cycling performance. The ALG, designed to control the release of nutrients, was compared to an isocaloric carbohydrate-only control (CON). Alginate encapsulation of CHOs has the potential to reduce the risk of carious lesions.
Methods: In a randomised cross-over clinical trial, 14 men completed a preliminary test over 2 experimental days separated by ~6 days. An experimental day consisted of an exercise bout (EX1) of cycling until exhaustion at W~73%, followed by 5 h of recovery and a subsequent time-to-exhaustion (TTE) performance test at W~65%. Subjects ingested either ALG (0.8 g CHO/kg/hr + 0.4 g PRO/kg/hr) or CON (1.2 g CHO/kg/hr) during the first 2 h of recovery.
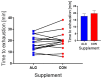
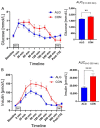
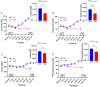
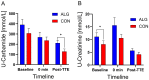
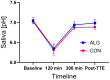
Results: Participants cycled on average 75.2 ± 5.9 min during EX1. Levels of plasma branched-chain amino acids decreased significantly after EX1, and increased significantly with the intake of ALG during the recovery period. During recovery, a significantly higher plasma insulin and glucose response was observed after intake of CON compared to ALG. Intake of ALG increased plasma glucagon, free fatty acids, and glycerol significantly. No differences were found in the TTE between the supplements (p = 0.13) nor in the pH of the subjects' saliva.
Conclusions: During the ALG supplement, plasma amino acids remained elevated during the recovery. Despite the 1/3 less CHO intake with ALG compared to CON, the TTE performance was similar after intake of either supplement.
Keywords: alginate encapsulation; athletic performance; carbohydrate; hydrogels; nutrition supplement; oral health; plant-based; protein; sports nutrition; time-to-exhaustion.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Hansen M., Bangsbo J., Jensen J., Krause-Jensen M., Bibby B.M., Sollie O., Hall U.A., Madsen K. Protein intake during training sessions has no effect on performance and recovery during a strenuous training camp for elite cyclists. J. Int. Soc. Sports Nutr. 2016;13:9. doi: 10.1186/s12970-016-0120-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

