Flowering and Runnering of Seasonal Strawberry under Different Photoperiods Are Affected by Intensity of Supplemental or Night-Interrupting Blue Light
- PMID: 38337908
- PMCID: PMC10857185
- DOI: 10.3390/plants13030375
Flowering and Runnering of Seasonal Strawberry under Different Photoperiods Are Affected by Intensity of Supplemental or Night-Interrupting Blue Light
Abstract
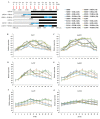
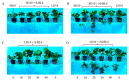
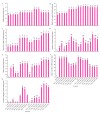
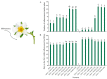
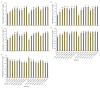
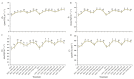
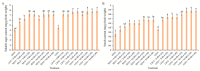
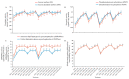
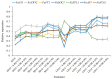
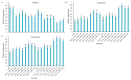
The strawberry (Fragaria × ananassa Duch.) "Sulhyang" is a typical seasonal flowering (SF) strawberry that produces flower buds in day lengths shorter than a critical limit (variable, but often defined as <12 h). There is a trade-off between photoperiod-controlled flowering and gibberellin (GA) signaling pathway-mediated runnering. Some related genes (such as CO, FT1, SOC1, and TFL1) participating in light signaling and circadian rhythm in plants are altered under blue light (BL). Sugars for flowering and runnering are mainly produced by photosynthetic carbon assimilation. The intensity of light could affect photosynthesis, thereby regulating flowering and runnering. Here, we investigated the effect of the intensity of supplemental blue light (S-BL) or night-interrupting blue light (NI-BL) in photoperiodic flowering and runnering regulation by applying 4 h of S-BL or NI-BL with either 0, 10, 20, 30, or 40 μmol·m-2·s-1 photosynthetic photon flux density (PPFD) in a 10 h short-day (SD10) (SD10 + S-BL4 or + NI-BL4 (0, 10, 20, 30, or 40)) or 14 h long-day (LD14) conditions (LD14 + S-BL4 or + NI-BL4 (0, 10, 20, 30, or 40)). Approximately 45 days after the photoperiodic light treatment, generally, whether S-BL or NI-BL, BL (20) was the most promotive in runnering, leading to more runners in both the LD and SD conditions. For flowering, except the treatment LD14 + S-BL, BL (20) was still the key light, either from BL (20) or BL (40), promoting flowering, especially when BL acted as the night-interrupting light, regardless of the photoperiod. At the harvest stage, larger numbers of inflorescences and runners were observed in the LD14 + NI-BL4 treatment, and the most were observed in the LD14 + NI-BL (20). Moreover, the SD10 + NI-BL4 was slightly inferior to the LD14 + NI-BL4 in increasing the numbers of inflorescences and runners, but it caused earlier flowering. Additionally, the circadian rhythm expression of flowering-related genes was affected differently by the S-BL and NI-BL. After the application of BL in LD conditions, the expression of an LD-specific floral activator FaFT1 was stimulated, while that of a flowering suppressor FaTFL1 was inhibited, resetting the balance of expression between these two opposite flowering regulators. The SD runnering was caused by BL in non-runnering SD conditions associated with the stimulation of two key genes that regulate runner formation in the GA pathway, FaGRAS32 and FaGA20ox4. In addition, the positive effects of BL on enhancing photosynthesis and carbohydrate production also provided an abundant energy supply for the flowering and runnering processes.
Keywords: GA pathway; carbohydrate accumulation; circadian rhythm; light intensity; photoperiodic response; photosynthetic efficiency; seasonal flowering; supplemental or night-interrupting blue light.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Staudt G. Taxonomic studies in the genus Fragaria typification of Fragaria species known at the time of Linnaeus. Can. J. Bot. 1962;40:869–886. doi: 10.1139/b62-081. - DOI
-
- Stauct G. The species of Fragaria, their taxonomy and geographical distribution; Proceedings of the International Strawberry Symposium 265; Cesena, Italy. 22–27 May 1988; pp. 23–34.
-
- Darrow G.M. The Strawberry. 1966. [(accessed on 30 October 2022)]. Available online: https://www.cabdirect.org/cabdirect/abstract/19680300487.
-
- Finn C.E., Retamales J.B., Lobos G.A., Hancock J.F. The Chilean strawberry (Fragaria chiloensis): Over 1000 years of domestication. HortScience. 2013;48:418–421. doi: 10.21273/HORTSCI.48.4.418. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

