Phase-Amplitude Coupling between Theta Rhythm and High-Frequency Oscillations in the Hippocampus of Pigeons during Navigation
- PMID: 38338082
- PMCID: PMC10854523
- DOI: 10.3390/ani14030439
Phase-Amplitude Coupling between Theta Rhythm and High-Frequency Oscillations in the Hippocampus of Pigeons during Navigation
Abstract
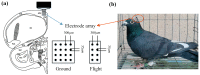
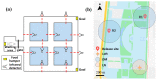
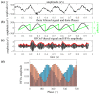
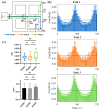
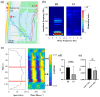
Navigation is a complex task in which the hippocampus (Hp), which plays an important role, may be involved in interactions between different frequency bands. However, little is known whether this cross-frequency interaction exists in the Hp of birds during navigation. Therefore, we examined the electrophysiological characteristics of hippocampal cross-frequency interactions of domestic pigeons (Columba livia domestica) during navigation. Two goal-directed navigation tasks with different locomotor modes were designed, and the local field potentials (LFPs) were recorded for analysis. We found that the amplitudes of high-frequency oscillations in Hp were dynamically modulated by the phase of co-occurring theta-band oscillations both during ground-based maze and outdoor flight navigation. The high-frequency amplitude sub-frequency bands modulated by the hippocampal theta phase were different at different tasks, and this process was independent of the navigation path and goal. These results suggest that phase-amplitude coupling (PAC) in the avian Hp may be more associated with the ongoing cognitive demands of navigational processes. Our findings contribute to the understanding of potential mechanisms of hippocampal PAC on multi-frequency informational interactions in avian navigation and provide valuable insights into cross-species evolution.
Keywords: cross-frequency interactions; flight; goal directed; local field potentials; theta.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

