Hypothalamic Neuromodulation of Hypothermia in Domestic Animals
- PMID: 38338158
- PMCID: PMC10854546
- DOI: 10.3390/ani14030513
Hypothalamic Neuromodulation of Hypothermia in Domestic Animals
Abstract
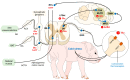
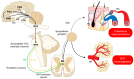
When an organism detects decreases in their core body temperature, the hypothalamus, the main thermoregulatory center, triggers compensatory responses. These responses include vasomotor changes to prevent heat loss and physiological mechanisms (e.g., shivering and non-shivering thermogenesis) for heat production. Both types of changes require the participation of peripheral thermoreceptors, afferent signaling to the spinal cord and hypothalamus, and efferent pathways to motor and/or sympathetic neurons. The present review aims to analyze the scientific evidence of the hypothalamic control of hypothermia and the central and peripheral changes that are triggered in domestic animals.
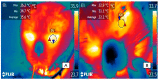
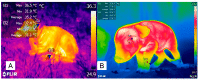
Keywords: brown adipose tissue thermogenesis; cold-defensive behaviors; cutaneous vasoconstriction; infrared thermography.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
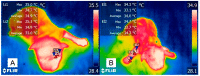

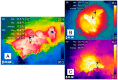
References
-
- Osilla E., Marsidi J., Sharma S. Physiology, Temperature Regulation. StatPearls Publishing; Tampa, FL, USA: 2018. - PubMed
-
- Krishnan G., Silpa M.V., Sejian V. Textbook of Veterinary Physiology. Springer Nature Singapore; Singapore: 2023. Environmental Physiology and Thermoregulation in Farm Animals; pp. 723–749.
-
- Oka T. Handbook of Clinical Neurology. Volume 157. Elsevier; Amsterdam, The Netherlands: 2018. Stress-Induced Hyperthermia and Hypothermia; pp. 599–621. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

