Radical Reactions in Organic Synthesis: Exploring in-, on-, and with-Water Methods
- PMID: 38338314
- PMCID: PMC10856544
- DOI: 10.3390/molecules29030569
Radical Reactions in Organic Synthesis: Exploring in-, on-, and with-Water Methods
Abstract
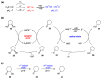
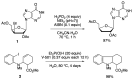
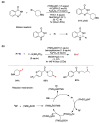
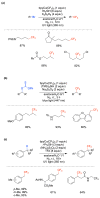
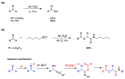
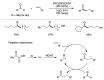
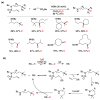
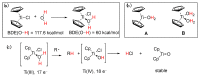
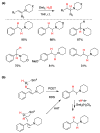
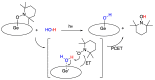
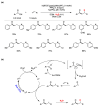
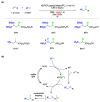
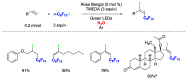
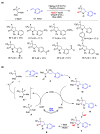
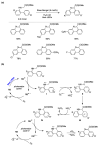
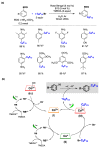
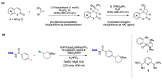
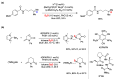
Radical reactions in water or aqueous media are important for organic synthesis, realizing high-yielding processes under non-toxic and environmentally friendly conditions. This overview includes (i) a general introduction to organic chemistry in water and aqueous media, (ii) synthetic approaches in, on, and with water as well as in heterogeneous phases, (iii) reactions of carbon-centered radicals with water (or deuterium oxide) activated through coordination with various Lewis acids, (iv) photocatalysis in water and aqueous media, and (v) synthetic applications bioinspired by naturally occurring processes. A wide range of chemical processes and synthetic strategies under different experimental conditions have been reviewed that lead to important functional group translocation and transformation reactions, leading to the preparation of complex molecules. These results reveal how water as a solvent/medium/reagent in radical chemistry has matured over the last two decades, with further discoveries anticipated in the near future.
Keywords: bioinspired reactions; on-water reactions; organic synthesis; photocatalysis; radical reactions; water and aqueous media; water coordination with Lewis acids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



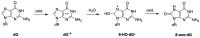
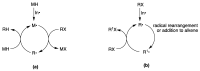


















Similar articles
-
Development and Applications of Water-Compatible Reactions: A Journey to Be Continued.Acc Chem Res. 2024 Jan 2;57(1):70-92. doi: 10.1021/acs.accounts.3c00555. Epub 2023 Dec 19. Acc Chem Res. 2024. PMID: 38112292
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
-
Lewis Base-Boryl Radicals Enabled Borylation Reactions and Selective Activation of Carbon-Heteroatom Bonds.Acc Chem Res. 2023 Jan 17;56(2):169-186. doi: 10.1021/acs.accounts.2c00752. Epub 2022 Dec 26. Acc Chem Res. 2023. PMID: 36571794
-
[Development of radical reactions in water aimed at environmentally benign synthetic reactions].Yakugaku Zasshi. 2004 Jun;124(6):311-9. doi: 10.1248/yakushi.124.311. Yakugaku Zasshi. 2004. PMID: 15170066 Review. Japanese.
-
Photoredox Catalysis for the Generation of Carbon Centered Radicals.Acc Chem Res. 2016 Sep 20;49(9):1924-36. doi: 10.1021/acs.accounts.6b00288. Epub 2016 Aug 16. Acc Chem Res. 2016. PMID: 27529633
Cited by
-
Impact of Transition-State Aromaticity on Selective Radical-Radical Coupling of Triarylimidazolyl Radicals.J Am Chem Soc. 2025 Jan 22;147(3):2559-2570. doi: 10.1021/jacs.4c14095. Epub 2024 Nov 27. J Am Chem Soc. 2025. PMID: 39604257 Free PMC article.
-
Design and application of a decatungstate-based ionic liquid photocatalyst for sustainable hydrogen atom transfer reactions.Green Chem. 2025 Jun 3;27(25):7660-7666. doi: 10.1039/d5gc02160j. eCollection 2025 Jun 23. Green Chem. 2025. PMID: 40485667 Free PMC article.
-
Aqueous Micellar Environment Impacts the Co-Catalyzed Phototransformation: A Case Study.J Am Chem Soc. 2024 Jul 24;146(29):19828-19838. doi: 10.1021/jacs.4c02682. Epub 2024 Jul 9. J Am Chem Soc. 2024. PMID: 39003762 Free PMC article.
-
Cyclopropanation with Non-Stabilized Carbenes via Ketyl Radicals.J Am Chem Soc. 2024 Aug 28;146(34):24009-24015. doi: 10.1021/jacs.4c07388. Epub 2024 Jul 24. J Am Chem Soc. 2024. PMID: 39049431 Free PMC article.
References
-
- Breslow R. Hydrophobic Effects on Simple Organic Reactions in Water. Acc. Chem. Res. 1991;24:159–164. doi: 10.1021/ar00006a001. - DOI
Publication types
LinkOut - more resources
Full Text Sources

