The Pharmaceutical Industry in 2023: An Analysis of FDA Drug Approvals from the Perspective of Molecules
- PMID: 38338330
- PMCID: PMC10856271
- DOI: 10.3390/molecules29030585
The Pharmaceutical Industry in 2023: An Analysis of FDA Drug Approvals from the Perspective of Molecules
Abstract
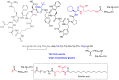
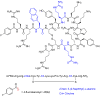
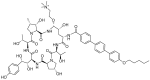
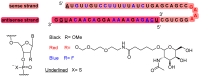
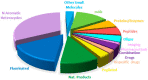
With the COVID-19 pandemic behind us, the U.S. Food and Drug Administration (FDA) has approved 55 new drugs in 2023, a figure consistent with the number authorized in the last five years (53 per year on average). Thus, 2023 marks the second-best yearly FDA harvest after 2018 (59 approvals) in all the series. Monoclonal antibodies (mAbs) continue to be the class of drugs with the most approvals, with an exceptional 12, a number that makes it the most outstanding year for this class. As in 2022, five proteins/enzymes have been approved in 2023. However, no antibody-drug conjugates (ADCs) have been released onto the market. With respect to TIDES (peptides and oligonucleotides), 2023 has proved a spectacular year, with a total of nine approvals, corresponding to five peptides and four oligonucleotides. Natural products continue to be the best source of inspiration for drug development, with 10 new products on the market. Three drugs in this year's harvest are pegylated, which may indicate the return of pegylation as a method to increase the half-lives of drugs after the withdrawal of peginesatide from the market in 2013. Following the trends in recent years, two bispecific drugs have been authorized in 2023. As in the preceding years, fluorine and/or N-aromatic heterocycles are present in most of the drugs. Herein, the 55 new drugs approved by the FDA in 2023 are analyzed exclusively on the basis of their chemical structure. They are classified as the following: biologics (antibodies, proteins/enzymes); TIDES (peptide and oligonucleotides); combined drugs; pegylated drugs; natural products; nitrogen aromatic heterocycles; fluorine-containing molecules; and other small molecules.
Keywords: TIDES; antibodies; biologics; chemical entities; fluorine-based drugs; imaging; natural products; new chemical entities; oligonucleotides; peptides.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
















References
-
- U.S. Food and Drug Administration (FDA) [(accessed on 1 January 2024)]; Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and....
-
- U.S. Food and Drug Administration (FDA) [(accessed on 1 January 2024)]; Available online: https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-f....
-
- U.S. Food and Drug Administration (FDA) [(accessed on 1 January 2024)]; Available online: https://www.fda.gov/vaccines-blood-biologics/development-approval-proces....
-
- Reata Pharmaceuticals has Been Acquired by Biogen. [(accessed on 1 January 2024)]. Available online: https://www.reatapharma.com/investors/news/news-details/2022/Reata-Pharm....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

