Degradation Product-Promoted Depolymerization Strategy for Chemical Recycling of Poly(bisphenol A carbonate)
- PMID: 38338384
- PMCID: PMC10856637
- DOI: 10.3390/molecules29030640
Degradation Product-Promoted Depolymerization Strategy for Chemical Recycling of Poly(bisphenol A carbonate)
Abstract
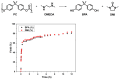
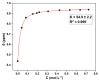
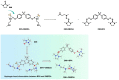
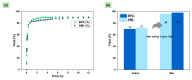
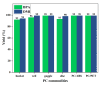
The accumulation of waste plastics has a severe impact on the environment, and therefore, the development of efficient chemical recycling methods has become an extremely important task. In this regard, a new strategy of degradation product-promoted depolymerization process was proposed. Using N,N'-dimethyl-ethylenediamine (DMEDA) as a depolymerization reagent, an efficient chemical recycling of poly(bisphenol A carbonate) (BPA-PC or PC) material was achieved under mild conditions. The degradation product 1,3-dimethyl-2-imidazolidinone (DMI) was proven to be a critical factor in facilitating the depolymerization process. This strategy does not require catalysts or auxiliary solvents, making it a truly green process. This method improves the recycling efficiency of PC and promotes the development of plastic reutilization.
Keywords: PC; bisphenol A; chemical recycling; depolymerization; plastic wastes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Using a natural chlorite as catalyst in chemical recycling of waste plastics: Hydrolytic depolymerization of poly-[bisphenol A carbonate] promoted by clinochlore.Waste Manag. 2021 Feb 1;120:642-649. doi: 10.1016/j.wasman.2020.10.031. Epub 2020 Nov 15. Waste Manag. 2021. PMID: 33208292
-
Chemical Recycling of Poly(bisphenol A carbonate) by Glycolysis under 1,8-Diazabicyclo[5.4.0]undec-7-ene Catalysis.ACS Omega. 2018 Jul 31;3(7):7261-7268. doi: 10.1021/acsomega.8b01123. Epub 2018 Jul 3. ACS Omega. 2018. PMID: 30087911 Free PMC article.
-
Recycling of End-of-Life Poly(bisphenol A carbonate) via Alkali Metal Halide-Catalyzed Phenolysis.ChemistryOpen. 2019 Jun 13;8(7):822-827. doi: 10.1002/open.201900149. eCollection 2019 Jul. ChemistryOpen. 2019. PMID: 31304075 Free PMC article.
-
Waste to Wealth: Chemical Recycling and Chemical Upcycling of Waste Plastics for a Great Future.ChemSusChem. 2021 Oct 5;14(19):4123-4136. doi: 10.1002/cssc.202100652. Epub 2021 Jun 1. ChemSusChem. 2021. PMID: 33998153 Review.
-
Mechanistic aspects of poly(ethylene terephthalate) recycling-toward enabling high quality sustainability decisions in waste management.Environ Sci Pollut Res Int. 2021 Aug;28(32):43074-43101. doi: 10.1007/s11356-021-14925-z. Epub 2021 Jun 19. Environ Sci Pollut Res Int. 2021. PMID: 34146328 Review.
References
-
- Potter H.V. Plastics and some recent applications. Nature. 1939;143:785–787. doi: 10.1038/143785a0. - DOI
-
- Bergmann M., Collard F., Fabres J., Gabrielsen G.W., Provencher J.F., Rochman C.M., Sebille E., Tekman M.B. Plastic pollution in the arctic. Nat. Rev. Earth Environ. 2022;3:323–337. doi: 10.1038/s43017-022-00279-8. - DOI
-
- Zhao X., Korey M., Li K., Copenhaver K., Tekinalp H., Celik S., Kalaitzidou K., Ruan R., Ragauskas A.J., Ozcan S. Plastic waste upcycling toward a circular economy. Chem. Eng. J. 2022;428:131928. doi: 10.1016/j.cej.2021.131928. - DOI
LinkOut - more resources
Full Text Sources

