Density Functional Theory Studies on the Chemical Reactivity of Allyl Mercaptan and Its Derivatives
- PMID: 38338412
- PMCID: PMC10856204
- DOI: 10.3390/molecules29030668
Density Functional Theory Studies on the Chemical Reactivity of Allyl Mercaptan and Its Derivatives
Abstract
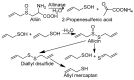
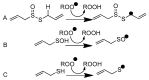
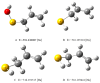
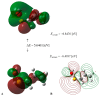
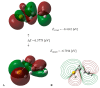
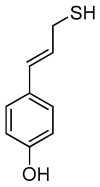
On the basis of density functional theory (DFT) at the B3LYP/cc-pVQZ level with the C-PCM solvation model, a comparative analysis of the reactivity of the garlic metabolites 2-propenesulfenic acid (PSA) and allyl mercaptan (AM, 2-propene-1-thiol) was performed. In particular, the thermodynamic descriptors (BDE, PA, ETE, AIP, PDE, and Gacidity) and global descriptors of chemical activity (ionization potential (IP), electron affinity (EA), chemical potential (μ), absolute electronegativity (χ), molecular hardness (η) and softness (S), electrophilicity index (ω), electro-donating (ω-) and electro-accepting (ω+) powers, and Ra and Rd indexes) were determined. The calculations revealed that PSA is more reactive than AM, but the latter may play a crucial role in the deactivation of free radicals due to its greater chemical stability and longer lifetime. The presence of a double bond in AM enables its polymerization, preserving the antiradical activity of the S-H group. This activity can be amplified by aryl-substituent-containing hydroxyl groups. The results of the calculations for the simplest phenol-AM derivative indicate that both the O-H and S-H moieties show greater antiradical activity in a vacuum and aqueous medium than the parent molecules. The results obtained prove that AM and its derivatives can be used not only as flavoring food additives but also as potent radical scavengers, protecting food, supplements, cosmetics, and drug ingredients from physicochemical decomposition caused by exogenous radicals.
Keywords: 2-propenesulfenic acid; DFT method; allyl mercaptan; chemical activity descriptors; garlic metabolites; thermodynamic descriptors.
Conflict of interest statement
The author declares no conflicts of interest.
Figures


Similar articles
-
Theoretical study on the radical scavenging activity of gallic acid.Heliyon. 2023 Jan 5;9(1):e12806. doi: 10.1016/j.heliyon.2023.e12806. eCollection 2023 Jan. Heliyon. 2023. PMID: 36691549 Free PMC article.
-
Density functional theory studies on molecular geometry, spectroscopy, HOMO-LUMO and reactivity descriptors of titanium(IV) and oxidozirconium(IV) complexes of phenylacetohydroxamic acid.J Comput Chem. 2022 Dec 5;43(31):2060-2071. doi: 10.1002/jcc.27004. Epub 2022 Sep 27. J Comput Chem. 2022. PMID: 36165982
-
Computational-based investigation of antioxidative potential polyphenolic compounds of Salvia officinalis L.: combined DFT and molecular docking approaches.J Mol Model. 2024 Feb 28;30(3):87. doi: 10.1007/s00894-024-05866-8. J Mol Model. 2024. PMID: 38416254
-
Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity.Toxicology. 2002 Aug 1;177(1):39-54. doi: 10.1016/s0300-483x(02)00194-4. Toxicology. 2002. PMID: 12126794 Review.
-
On the Periodicity of the Information Theory and Conceptual DFT-Based Reactivity Descriptors.J Phys Chem A. 2022 Oct 6;126(39):6801-6813. doi: 10.1021/acs.jpca.2c04106. Epub 2022 Sep 26. J Phys Chem A. 2022. PMID: 36154006 Review.
Cited by
-
A Theoretical Investigation into the Oligomer Structure of Carbon Dots Formed from Small-Molecule Precursors.Molecules. 2024 Jun 19;29(12):2920. doi: 10.3390/molecules29122920. Molecules. 2024. PMID: 38930988 Free PMC article.
-
Theoretical Insight into Psittacofulvins and Their Derivatives.Molecules. 2024 Jun 10;29(12):2760. doi: 10.3390/molecules29122760. Molecules. 2024. PMID: 38930826 Free PMC article.
-
Enhancing Bioactivity through the Transfer of the 2-(Hydroxymethoxy)Vinyl Moiety: Application in the Modification of Tyrosol and Hinokitiol.Molecules. 2024 Jul 21;29(14):3414. doi: 10.3390/molecules29143414. Molecules. 2024. PMID: 39064992 Free PMC article.
References
-
- Hiramatsu K., Tsuneyoshi T., Ogawa T., Morihara N. Aged garlic extract enhances heme oxygenase-1 and glutamate-cysteine ligase modifier subunit expression via the nuclear factor erythroid 2-related factor 2-antioxidant response element signaling pathway in human endothelial cells. Nutr. Res. 2016;36:143–149. doi: 10.1016/j.nutres.2015.09.018. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

