Fingolimod Inhibits Exopolysaccharide Production and Regulates Relevant Genes to Eliminate the Biofilm of K. pneumoniae
- PMID: 38338675
- PMCID: PMC10855953
- DOI: 10.3390/ijms25031397
Fingolimod Inhibits Exopolysaccharide Production and Regulates Relevant Genes to Eliminate the Biofilm of K. pneumoniae
Abstract
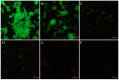
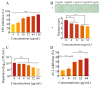
Klebsiella pneumoniae (K. pneumoniae) exhibits the ability to form biofilms as a means of adapting to its adverse surroundings. K. pneumoniae in this biofilm state demonstrates remarkable resistance, evades immune system attacks, and poses challenges for complete eradication, thereby complicating clinical anti-infection efforts. Moreover, the precise mechanisms governing biofilm formation and disruption remain elusive. Recent studies have discovered that fingolimod (FLD) exhibits biofilm properties against Gram-positive bacteria. Therefore, the antibiofilm properties of FLD were evaluated against multidrug-resistant (MDR) K. pneumoniae in this study. The antibiofilm activity of FLD against K. pneumoniae was assessed utilizing the Alamar Blue assay along with confocal laser scanning microscopy (CLSM), scanning electron microscopy (SEM), and crystal violet (CV) staining. The results showed that FLD effectively reduced biofilm formation, exopolysaccharide (EPS), motility, and bacterial abundance within K. pneumoniae biofilms without impeding its growth and metabolic activity. Furthermore, the inhibitory impact of FLD on the production of autoinducer-2 (AI-2) signaling molecules was identified, thereby demonstrating its notable anti-quorum sensing (QS) properties. The results of qRT-PCR analysis demonstrated that FLD significantly decreased the expression of genes associated with the efflux pump gene (AcrB, kexD, ketM, kdeA, and kpnE), outer membrane (OM) porin proteins (OmpK35, OmpK36), the quorum-sensing (QS) system (luxS), lipopolysaccharide (LPS) production (wzm), and EPS production (pgaA). Simultaneously, FLD exhibited evident antibacterial synergism, leading to an increased survival rate of G. mellonella infected with MDR K. pneumoniae. These findings suggested that FLD has substantial antibiofilm properties and synergistic antibacterial potential for colistin in treating K. pneumoniae infections.
Keywords: K. pneumoniae; antibacterial; biofilm; fingolimod (FLD); synergistic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains.J Appl Microbiol. 2017 Oct;123(4):1003-1018. doi: 10.1111/jam.13533. Epub 2017 Aug 25. J Appl Microbiol. 2017. PMID: 28731269
-
Investigation of LuxS-mediated quorum sensing in Klebsiella pneumoniae.J Med Microbiol. 2020 Mar;69(3):402-413. doi: 10.1099/jmm.0.001148. J Med Microbiol. 2020. PMID: 32223838 Free PMC article.
-
Antibacterial, anti-efflux, anti-biofilm, anti-slime (exopolysaccharide) production and urease inhibitory efficacies of novel synthesized gold nanoparticles coated Anthemis atropatana extract against multidrug- resistant Klebsiella pneumoniae strains.Arch Microbiol. 2020 Oct;202(8):2105-2115. doi: 10.1007/s00203-020-01930-y. Epub 2020 Jun 4. Arch Microbiol. 2020. PMID: 32500253
-
Promising strategies for future treatment of Klebsiella pneumoniae biofilms.Future Microbiol. 2020 Jan;15:63-79. doi: 10.2217/fmb-2019-0180. Epub 2020 Feb 12. Future Microbiol. 2020. PMID: 32048525 Review.
-
Klebsiella pneumoniae Biofilms and Their Role in Disease Pathogenesis.Front Cell Infect Microbiol. 2022 May 11;12:877995. doi: 10.3389/fcimb.2022.877995. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35646720 Free PMC article. Review.
Cited by
-
Unveiling the potential of spirulina algal extract as promising antibacterial and antibiofilm agent against carbapenem-resistant Klebsiella pneumoniae: in vitro and in vivo study.Microb Cell Fact. 2025 Jan 5;24(1):7. doi: 10.1186/s12934-024-02619-3. Microb Cell Fact. 2025. PMID: 39755644 Free PMC article.
-
FTY720 Reduces the Biomass of Biofilms in Pseudomonas aeruginosa in a Dose-Dependent Manner.Antibiotics (Basel). 2024 Jul 4;13(7):621. doi: 10.3390/antibiotics13070621. Antibiotics (Basel). 2024. PMID: 39061303 Free PMC article.
-
Study on the invitro synergistic susceptibility and biofilm inhibition mechanism of ceftazidime-avibactam combined with aztreonam against carbapenem-resistant Klebsiella pneumoniae.Front Microbiol. 2025 Mar 20;16:1542029. doi: 10.3389/fmicb.2025.1542029. eCollection 2025. Front Microbiol. 2025. PMID: 40182285 Free PMC article.
References
-
- Allen I.C. Using Klebsiella pneumoniae to Model Acute Lung Inflammation in Mice. Volume 1960. Springer; New York, NY, USA: 2019. pp. 169–180. - PubMed
-
- Ramos-Castaneda J.A., Ruano-Ravina A., Barbosa-Lorenzo R., Paillier-Gonzalez J.E., Saldana-Campos J.C., Salinas D.F., Lemos-Luengas E.V. Mortality due to KPC carbapenemase-producing Klebsiella pneumoniae infections: Systematic review and meta-analysis: Mortality due to KPC Klebsiella pneumoniae infections. J. Infect. 2018;76:438–448. doi: 10.1016/j.jinf.2018.02.007. - DOI - PubMed
-
- Veselova V.O., Plyuta V.A., Kostrov A.N., Vtyurina D.N., Abramov V.O., Abramova A.V., Voitov Y.I., Padiy D.A., Thu V.T.H., Hue L.T., et al. Long-term antimicrobial performance of textiles coated with ZnO and TiO2 nanoparticles in a tropical climate. J. Funct. Biomater. 2022;13:233. doi: 10.3390/jfb13040233. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

