Unraveling the Role of Ras Homolog Enriched in Brain (Rheb1 and Rheb2): Bridging Neuronal Dynamics and Cancer Pathogenesis through Mechanistic Target of Rapamycin Signaling
- PMID: 38338768
- PMCID: PMC10855792
- DOI: 10.3390/ijms25031489
Unraveling the Role of Ras Homolog Enriched in Brain (Rheb1 and Rheb2): Bridging Neuronal Dynamics and Cancer Pathogenesis through Mechanistic Target of Rapamycin Signaling
Abstract
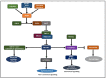
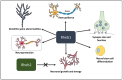
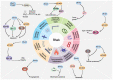
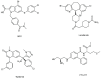
Ras homolog enriched in brain (Rheb1 and Rheb2), small GTPases, play a crucial role in regulating neuronal activity and have gained attention for their implications in cancer development, particularly in breast cancer. This study delves into the intricate connection between the multifaceted functions of Rheb1 in neurons and cancer, with a specific focus on the mTOR pathway. It aims to elucidate Rheb1's involvement in pivotal cellular processes such as proliferation, apoptosis resistance, migration, invasion, metastasis, and inflammatory responses while acknowledging that Rheb2 has not been extensively studied. Despite the recognized associations, a comprehensive understanding of the intricate interplay between Rheb1 and Rheb2 and their roles in both nerve and cancer remains elusive. This review consolidates current knowledge regarding the impact of Rheb1 on cancer hallmarks and explores the potential of Rheb1 as a therapeutic target in cancer treatment. It emphasizes the necessity for a deeper comprehension of the molecular mechanisms underlying Rheb1-mediated oncogenic processes, underscoring the existing gaps in our understanding. Additionally, the review highlights the exploration of Rheb1 inhibitors as a promising avenue for cancer therapy. By shedding light on the complicated roles between Rheb1/Rheb2 and cancer, this study provides valuable insights to the scientific community. These insights are instrumental in guiding the identification of novel targets and advancing the development of effective therapeutic strategies for treating cancer.
Keywords: Rheb1; Rheb1 inhibitors; Rheb2; cancer; hallmarks; mTOR; neurons.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Rheb1 is required for mTORC1 and myelination in postnatal brain development.Dev Cell. 2011 Jan 18;20(1):97-108. doi: 10.1016/j.devcel.2010.11.020. Dev Cell. 2011. PMID: 21238928 Free PMC article.
-
Genetic deletion of Rheb1 in the brain reduces food intake and causes hypoglycemia with altered peripheral metabolism.Int J Mol Sci. 2014 Jan 21;15(1):1499-510. doi: 10.3390/ijms15011499. Int J Mol Sci. 2014. PMID: 24451134 Free PMC article.
-
Rheb1 deletion in myeloid cells aggravates OVA-induced allergic inflammation in mice.Sci Rep. 2017 Feb 22;7:42655. doi: 10.1038/srep42655. Sci Rep. 2017. PMID: 28225024 Free PMC article.
-
Perspectives of RAS and RHEB GTPase Signaling Pathways in Regenerating Brain Neurons.Int J Mol Sci. 2018 Dec 14;19(12):4052. doi: 10.3390/ijms19124052. Int J Mol Sci. 2018. PMID: 30558189 Free PMC article. Review.
-
Rheb/mTOR activation and regulation in cancer: novel treatment strategies beyond rapamycin.Curr Drug Targets. 2011 Jul 1;12(8):1223-31. doi: 10.2174/138945011795906589. Curr Drug Targets. 2011. PMID: 21561413 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

