Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression
- PMID: 38338783
- PMCID: PMC10855860
- DOI: 10.3390/ijms25031504
Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression
Abstract
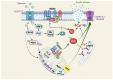
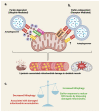
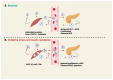
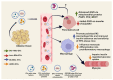
Type 2 diabetes (T2D) is a heterogenous disease, and conventionally, peripheral insulin resistance (IR) was thought to precede islet β-cell dysfunction, promoting progression from prediabetes to T2D. New evidence suggests that T2D-lean individuals experience early β-cell dysfunction without significant IR. Regardless of the primary event (i.e., IR vs. β-cell dysfunction) that contributes to dysglycemia, significant early-onset oxidative damage and mitochondrial dysfunction in multiple metabolic tissues may be a driver of T2D onset and progression. Oxidative stress, defined as the generation of reactive oxygen species (ROS), is mediated by hyperglycemia alone or in combination with lipids. Physiological oxidative stress promotes inter-tissue communication, while pathological oxidative stress promotes inter-tissue mis-communication, and new evidence suggests that this is mediated via extracellular vesicles (EVs), including mitochondria containing EVs. Under metabolic-related stress conditions, EV-mediated cross-talk between β-cells and skeletal muscle likely trigger mitochondrial anomalies leading to prediabetes and T2D. This article reviews the underlying molecular mechanisms in ROS-related pathogenesis of prediabetes, including mitophagy and mitochondrial dynamics due to oxidative stress. Further, this review will describe the potential of various therapeutic avenues for attenuating oxidative damage, reversing prediabetes and preventing progression to T2D.
Keywords: extracellular vesicles; insulin resistance; islet beta cells; mitochondria; oxidative stress; prediabetes; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cefalu W.T., Andersen D.K., Arreaza-Rubín G., Pin C.L., Sato S., Verchere C.B., Woo M., Rosenblum N.D. Heterogeneity of Diabetes: β-Cells, Phenotypes, and Precision Medicine: Proceedings of an International Symposium of the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes and the U.S. National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes Care. 2022;45:3–22. doi: 10.2337/dci21-0051. - DOI - PMC - PubMed
-
- International Diabetes Federation . Diabetes Atlas. 10th ed. International Diabetes Federation; Brussels, Belgium: 2021.
-
- Wesolowska-Andersen A., Brorsson C.A., Bizzotto R., Mari A., Tura A., Koivula R., Mahajan A., Vinuela A., Tajes J.F., Sharma S., et al. Four groups of type 2 diabetes contribute to the etiological and clinical heterogeneity in newly diagnosed individuals: An IMI DIRECT study. Cell Rep. Med. 2022;3:100477. doi: 10.1016/j.xcrm.2021.100477. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

