Mitochondrial Signaling, the Mechanisms of AKI-to-CKD Transition and Potential Treatment Targets
- PMID: 38338797
- PMCID: PMC10855342
- DOI: 10.3390/ijms25031518
Mitochondrial Signaling, the Mechanisms of AKI-to-CKD Transition and Potential Treatment Targets
Abstract
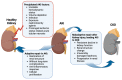
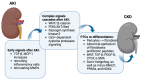
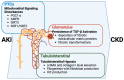
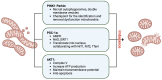
Acute kidney injury (AKI) is increasing in prevalence and causes a global health burden. AKI is associated with significant mortality and can subsequently develop into chronic kidney disease (CKD). The kidney is one of the most energy-demanding organs in the human body and has a role in active solute transport, maintenance of electrochemical gradients, and regulation of fluid balance. Renal proximal tubular cells (PTCs) are the primary segment to reabsorb and secrete various solutes and take part in AKI initiation. Mitochondria, which are enriched in PTCs, are the main source of adenosine triphosphate (ATP) in cells as generated through oxidative phosphorylation. Mitochondrial dysfunction may result in reactive oxygen species (ROS) production, impaired biogenesis, oxidative stress multiplication, and ultimately leading to cell death. Even though mitochondrial damage and malfunction have been observed in both human kidney disease and animal models of AKI and CKD, the mechanism of mitochondrial signaling in PTC for AKI-to-CKD transition remains unknown. We review the recent findings of the development of AKI-to-CKD transition with a focus on mitochondrial disorders in PTCs. We propose that mitochondrial signaling is a key mechanism of the progression of AKI to CKD and potential targeting for treatment.
Keywords: AKI; AKT1; CKD; acute kidney injury; chronic kidney disease; mitochondria.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and Its Therapeutic Potential.Int J Mol Sci. 2021 Oct 19;22(20):11253. doi: 10.3390/ijms222011253. Int J Mol Sci. 2021. PMID: 34681922 Free PMC article. Review.
-
Mitochondrial dysfunction and the AKI-to-CKD transition.Am J Physiol Renal Physiol. 2020 Dec 1;319(6):F1105-F1116. doi: 10.1152/ajprenal.00285.2020. Epub 2020 Oct 19. Am J Physiol Renal Physiol. 2020. PMID: 33073587 Review.
-
Intermedin protects peritubular capillaries by inhibiting eNOS uncoupling through AMPK/GTPCH-I/BH4 pathway and alleviate CKD following AKI.Free Radic Biol Med. 2025 Jul;234:72-85. doi: 10.1016/j.freeradbiomed.2025.04.015. Epub 2025 Apr 12. Free Radic Biol Med. 2025. PMID: 40228707
-
Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD.J Am Soc Nephrol. 2017 Oct;28(10):2856-2865. doi: 10.1681/ASN.2017030247. Epub 2017 Aug 4. J Am Soc Nephrol. 2017. PMID: 28778860 Free PMC article. Review.
-
Pathophysiological Role of Organelle Stress/Crosstalk in AKI-to-CKD Transition.Semin Nephrol. 2019 Nov;39(6):581-588. doi: 10.1016/j.semnephrol.2019.10.007. Semin Nephrol. 2019. PMID: 31836040 Review.
Cited by
-
The Application and Molecular Mechanisms of Mitochondria-Targeted Antioxidants in Chemotherapy-Induced Cardiac Injury.Curr Issues Mol Biol. 2025 Mar 7;47(3):176. doi: 10.3390/cimb47030176. Curr Issues Mol Biol. 2025. PMID: 40136430 Free PMC article. Review.
-
Multiple roles of mitochondrial autophagy receptor FUNDC1 in mitochondrial events and kidney disease.Front Cell Dev Biol. 2024 Oct 9;12:1453365. doi: 10.3389/fcell.2024.1453365. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39445333 Free PMC article. Review.
-
Proteomic analysis of laser captured tubular tissues reveals complement activation and mitochondrial dysfunction in autoimmune related kidney diseases.Sci Rep. 2024 Aug 20;14(1):19311. doi: 10.1038/s41598-024-70209-9. Sci Rep. 2024. PMID: 39164435 Free PMC article.
References
-
- Hoste E.A.J., Bagshaw S.M., Bellomo R., Cely C.M., Colman R., Cruz D.N., Edipidis K., Forni L.G., Gomersall C.D., Govil D., et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensiv. Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

