TRPV4 Channels Promote Pathological, but Not Physiological, Cardiac Remodeling through the Activation of Calcineurin/NFAT and TRPC6
- PMID: 38338818
- PMCID: PMC10855372
- DOI: 10.3390/ijms25031541
TRPV4 Channels Promote Pathological, but Not Physiological, Cardiac Remodeling through the Activation of Calcineurin/NFAT and TRPC6
Abstract
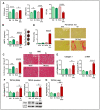
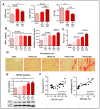
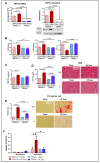
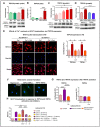
TRPV4 channels, which respond to mechanical activation by permeating Ca2+ into the cell, may play a pivotal role in cardiac remodeling during cardiac overload. Our study aimed to investigate TRPV4 involvement in pathological and physiological remodeling through Ca2+-dependent signaling. TRPV4 expression was assessed in heart failure (HF) models, induced by isoproterenol infusion or transverse aortic constriction, and in exercise-induced adaptive remodeling models. The impact of genetic TRPV4 inhibition on HF was studied by echocardiography, histology, gene and protein analysis, arrhythmia inducibility, Ca2+ dynamics, calcineurin (CN) activity, and NFAT nuclear translocation. TRPV4 expression exclusively increased in HF models, strongly correlating with fibrosis. Isoproterenol-administered transgenic TRPV4-/- mice did not exhibit HF features. Cardiac fibroblasts (CFb) from TRPV4+/+ animals, compared to TRPV4-/-, displayed significant TRPV4 overexpression, elevated Ca2+ influx, and enhanced CN/NFATc3 pathway activation. TRPC6 expression paralleled that of TRPV4 in all models, with no increase in TRPV4-/- mice. In cultured CFb, the activation of TRPV4 by GSK1016790A increased TRPC6 expression, which led to enhanced CN/NFATc3 activation through synergistic action of both channels. In conclusion, TRPV4 channels contribute to pathological remodeling by promoting fibrosis and inducing TRPC6 upregulation through the activation of Ca2+-dependent CN/NFATc3 signaling. These results pose TRPV4 as a primary mediator of the pathological response.
Keywords: TRP; TRPC6; TRPV4; calcium; exercise; heart failure; mechanoreceptors; mechanotransduction; pathological remodeling; physiological remodeling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- McMullen J.R., Shioi T., Zhang L., Tarnavski O., Sherwood M.C., Kang P.M., Izumo S. Phosphoinositide 3-Kinase(P110α) Plays a Critical Role for the Induction of Physiological, but Not Pathological, Cardiac Hypertrophy. Proc. Natl. Acad. Sci. USA. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

