A Blood Test for the Diagnosis of Multiple Sclerosis
- PMID: 38338973
- PMCID: PMC10855725
- DOI: 10.3390/ijms25031696
A Blood Test for the Diagnosis of Multiple Sclerosis
Abstract
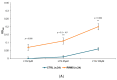
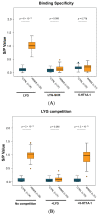
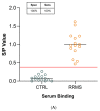
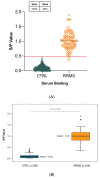
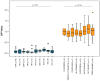
Multiple sclerosis (MS) is an autoimmune chronic disease characterized by inflammation and demyelination of the central nervous system (CNS). Despite numerous studies conducted, valid biomarkers enabling a definitive diagnosis of MS are not yet available. The aim of our study was to identify a marker from a blood sample to ease the diagnosis of MS. In this study, since there is evidence connecting the serotonin pathway to MS, we used an ELISA (Enzyme-Linked Immunosorbent Assay) to detect serum MS-specific auto-antibodies (auto-Ab) against the extracellular loop 1 (ECL-1) of the 5-hydroxytryptamine (5-HT) receptor subtype 2A (5-HT2A). We utilized an ELISA format employing poly-D-lysine as a pre-coating agent. The binding of 208 serum samples from controls, both healthy and pathological, and of 104 serum samples from relapsing-remitting MS (RRMS) patients was tested. We observed that the serum-binding activity in control cohort sera, including those with autoimmune and neurological diseases, was ten times lower compared to the RRMS patient cohort (p = 1.2 × 10-47), with a sensitivity and a specificity of 98% and 100%, respectively. These results show that in the serum of patients with MS there are auto-Ab against the serotonin receptor type 2A which can be successfully used in the diagnosis of MS due to their high sensitivity and specificity.
Keywords: 5-HT2A receptor; ELISA assay; blood serum; diagnosis; multiple sclerosis; peptides.
Conflict of interest statement
R.P. has received research grants from PRINDEX S.r.l.; P.G., M.S. and R.P are equity owners in PRINDEX S.r.l.; P.G., G.L.R., S.C. and S.D. have been involved as consultants in PRINDEX S.r.l.; L.M.P., M.S. and R.P. are the inventors of USA patent 10633427 and EPO patent 3109257.
Figures


References
-
- Freedman M.S., Comi G., De Stefano N., Barkhof F., Polman C.H., Uitdehaag B.M., Lehr L., Stubinski B., Kappos L. Moving toward earlier treatment of multiple sclerosis: Findings from a decade of clinical trials and implications for clinical practice. Mult. Scler. Relat. Disord. 2014;3:147–155. doi: 10.1016/j.msard.2013.07.001. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

