In-Depth Genomic Analysis: The New Challenge in Congenital Heart Disease
- PMID: 38339013
- PMCID: PMC10855915
- DOI: 10.3390/ijms25031734
In-Depth Genomic Analysis: The New Challenge in Congenital Heart Disease
Abstract
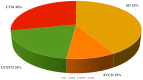
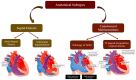
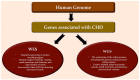
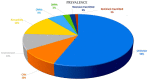
The use of next-generation sequencing has provided new insights into the causes and mechanisms of congenital heart disease (CHD). Examinations of the whole exome sequence have detected detrimental gene variations modifying single or contiguous nucleotides, which are characterised as pathogenic based on statistical assessments of families and correlations with congenital heart disease, elevated expression during heart development, and reductions in harmful protein-coding mutations in the general population. Patients with CHD and extracardiac abnormalities are enriched for gene classes meeting these criteria, supporting a common set of pathways in the organogenesis of CHDs. Single-cell transcriptomics data have revealed the expression of genes associated with CHD in specific cell types, and emerging evidence suggests that genetic mutations disrupt multicellular genes essential for cardiogenesis. Metrics and units are being tracked in whole-genome sequencing studies.
Keywords: congenital heart disease; copy number variants; de novo mutations; deletion; first heart field; gene variants; loss-of-function variant; second heart field; whole-exome sequencing; whole-genome sequencing.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

Similar articles
-
Genomic frontiers in congenital heart disease.Nat Rev Cardiol. 2022 Jan;19(1):26-42. doi: 10.1038/s41569-021-00587-4. Epub 2021 Jul 16. Nat Rev Cardiol. 2022. PMID: 34272501 Free PMC article. Review.
-
Increased frequency of de novo copy number variants in congenital heart disease by integrative analysis of single nucleotide polymorphism array and exome sequence data.Circ Res. 2014 Oct 24;115(10):884-896. doi: 10.1161/CIRCRESAHA.115.304458. Epub 2014 Sep 9. Circ Res. 2014. PMID: 25205790 Free PMC article. Clinical Trial.
-
Molecular Genetics and Complex Inheritance of Congenital Heart Disease.Genes (Basel). 2021 Jun 30;12(7):1020. doi: 10.3390/genes12071020. Genes (Basel). 2021. PMID: 34209044 Free PMC article. Review.
-
Clinical and genetic findings in patients with congenital cataract and heart diseases.Orphanet J Rare Dis. 2021 May 31;16(1):242. doi: 10.1186/s13023-021-01873-7. Orphanet J Rare Dis. 2021. PMID: 34059112 Free PMC article.
-
Integration of Large-Scale Genomic Data Sources With Evolutionary History Reveals Novel Genetic Loci for Congenital Heart Disease.Circ Genom Precis Med. 2019 Oct;12(10):442-451. doi: 10.1161/CIRCGEN.119.002694. Epub 2019 Oct 15. Circ Genom Precis Med. 2019. PMID: 31613678 Free PMC article.
Cited by
-
Maternal di(2-ethylhexyl) phthalate exposure increases the risk of congenital heart disease in mice offspring.Pediatr Res. 2025 Mar 15. doi: 10.1038/s41390-025-03997-z. Online ahead of print. Pediatr Res. 2025. PMID: 40089607
-
Toll-like Receptors 1, 3 and 7 Activate Distinct Genetic Features of NF-κB Signaling and γ-Protocadherin Expression in Human Cardiac Fibroblasts.Inflammation. 2025 Jan 20. doi: 10.1007/s10753-025-02238-z. Online ahead of print. Inflammation. 2025. PMID: 39828779
-
Clinical Genetic and Genomic Testing in Congenital Heart Disease and Cardiomyopathy.J Clin Med. 2024 Apr 26;13(9):2544. doi: 10.3390/jcm13092544. J Clin Med. 2024. PMID: 38731073 Free PMC article. Review.
-
Current and future diagnostics of congenital heart disease (CHD).Med Genet. 2025 Apr 8;37(2):95-102. doi: 10.1515/medgen-2025-2008. eCollection 2025 Jun. Med Genet. 2025. PMID: 40207043 Free PMC article.
References
-
- Dilli D., Akduman H., Zenciroğlu A., Çetinkaya M., Okur N., Turan Ö., Özlü F., Çalkavur Ş., Demirel G., Koksal N., et al. Neonatal Outcomes of Critical Congenital Heart Defects: A Multicenter Epidemiological Study of Turkish Neonatal Society: Neonatal Outcomes of CCHD. Pediatr. Cardiol. 2024;45:257–271. doi: 10.1007/s00246-023-03362-z. - DOI - PubMed
-
- Hossin M.Z., de la Cruz L.F., McKay K.A., Oberlander T.F., Sandström A., Razaz N. Association of pre-existing maternal cardiovascular diseases with neurodevelopmental disorders in offspring: A cohort study in Sweden and British Columbia, Canada. Int. J. Epidemiol. 2023:dyad184. doi: 10.1093/ije/dyad184. - DOI - PMC - PubMed
-
- Bakker M.K., Bergman J.E.H., Krikov S., Amar E., Cocchi G., Cragan J., de Walle H.E.K., Gatt M., Groisman B., Liu S., et al. Prenatal diagnosis and prevalence of critical congenital heart defects: An international retrospective cohort study. BMJ Open. 2019;9:e028139. doi: 10.1136/bmjopen-2018-028139. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

