Interplay between Microbiota and γδ T Cells: Insights into Immune Homeostasis and Neuro-Immune Interactions
- PMID: 38339023
- PMCID: PMC10855551
- DOI: 10.3390/ijms25031747
Interplay between Microbiota and γδ T Cells: Insights into Immune Homeostasis and Neuro-Immune Interactions
Abstract
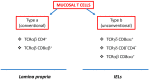
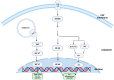
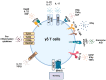
The gastrointestinal (GI) tract of multicellular organisms, especially mammals, harbors a symbiotic commensal microbiota with diverse microorganisms including bacteria, fungi, viruses, and other microbial and eukaryotic species. This microbiota exerts an important role on intestinal function and contributes to host health. The microbiota, while benefiting from a nourishing environment, is involved in the development, metabolism and immunity of the host, contributing to the maintenance of homeostasis in the GI tract. The immune system orchestrates the maintenance of key features of host-microbe symbiosis via a unique immunological network that populates the intestinal wall with different immune cell populations. Intestinal epithelium contains lymphocytes in the intraepithelial (IEL) space between the tight junctions and the basal membrane of the gut epithelium. IELs are mostly CD8+ T cells, with the great majority of them expressing the CD8αα homodimer, and the γδ T cell receptor (TCR) instead of the αβ TCR expressed on conventional T cells. γδ T cells play a significant role in immune surveillance and tissue maintenance. This review provides an overview of how the microbiota regulates γδ T cells and the influence of microbiota-derived metabolites on γδ T cell responses, highlighting their impact on immune homeostasis. It also discusses intestinal neuro-immune regulation and how γδ T cells possess the ability to interact with both the microbiota and brain.
Keywords: dysbiosis; inflammation; microbiota; neurotransmitters; γδ T cells.
Conflict of interest statement
All authors declare that this review article was created in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

