Clinical Characteristics and Local Histopathological Modulators of Endometriosis and Its Progression
- PMID: 38339066
- PMCID: PMC10855449
- DOI: 10.3390/ijms25031789
Clinical Characteristics and Local Histopathological Modulators of Endometriosis and Its Progression
Abstract
Endometriosis (E) and adenomyosis (A) are associated with a wide spectrum of symptoms and may present various histopathological transformations, such as the presence of hyperplasia, atypia, and malignant transformation occurring under the influence of local inflammatory, vascular and hormonal factors and by the alteration of tumor suppressor proteins and the inhibition of cell apoptosis, with an increased degree of lesion proliferation.
Material and methods: This retrospective study included 243 patients from whom tissue with E/A or normal control uterine tissue was harvested and stained by histochemical and classical immunohistochemical staining. We assessed the symptomatology of the patients, the structure of the ectopic epithelium and the presence of neovascularization, hormone receptors, inflammatory cells and oncoproteins involved in lesion development. Atypical areas were analyzed using multiple immunolabeling techniques.
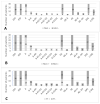
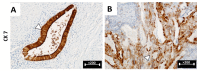
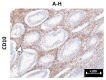
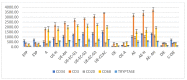
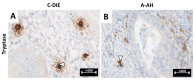
Results: The cytokeratin (CK) CK7+/CK20- expression profile was present in E foci and differentiated them from digestive metastases. The neovascularization marker cluster of differentiation (CD) 34+ was increased, especially in areas with malignant transformation of E or A foci. T:CD3+ lymphocytes, B:CD20+ lymphocytes, CD68+ macrophages and tryptase+ mast cells were abundant, especially in cases associated with malignant transformation, being markers of the proinflammatory microenvironment. In addition, we found a significantly increased cell division index (Ki67+), with transformation and inactivation of tumor suppressor genes p53, B-cell lymphoma 2 (BCL-2) and Phosphatase and tensin homolog (PTEN) in areas with E/A-transformed malignancy.
Conclusions: Proinflammatory/vascular/hormonal changes trigger E/A progression and the onset of cellular atypia and malignant transformation, exacerbating symptoms, especially local pain and vaginal bleeding. These triggers may represent future therapeutic targets.
Keywords: adenomyosis; endometriosis; multiple immunohistochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Schorage J.O., Schaffer J.J., Halvorson L.M., Hoffman B.L., Bradshaw K.D., Cunningham F.G. Williams Gynecology. McGraw Hill Medical; New York, NY, USA: 2008.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

