Metabolic Insights into Caffeine's Anti-Adipogenic Effects: An Exploration through Intestinal Microbiota Modulation in Obesity
- PMID: 38339081
- PMCID: PMC10855966
- DOI: 10.3390/ijms25031803
Metabolic Insights into Caffeine's Anti-Adipogenic Effects: An Exploration through Intestinal Microbiota Modulation in Obesity
Abstract
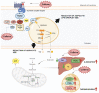
Obesity, a chronic condition marked by the excessive accumulation of adipose tissue, not only affects individual well-being but also significantly inflates healthcare costs. The physiological excess of fat manifests as triglyceride (TG) deposition within adipose tissue, with white adipose tissue (WAT) expansion via adipocyte hyperplasia being a key adipogenesis mechanism. As efforts intensify to address this global health crisis, understanding the complex interplay of contributing factors becomes critical for effective public health interventions and improved patient outcomes. In this context, gut microbiota-derived metabolites play an important role in orchestrating obesity modulation. Microbial lipopolysaccharides (LPS), secondary bile acids (BA), short-chain fatty acids (SCFAs), and trimethylamine (TMA) are the main intestinal metabolites in dyslipidemic states. Emerging evidence highlights the microbiota's substantial role in influencing host metabolism and subsequent health outcomes, presenting new avenues for therapeutic strategies, including polyphenol-based manipulations of these microbial populations. Among various agents, caffeine emerges as a potent modulator of metabolic pathways, exhibiting anti-inflammatory, antioxidant, and obesity-mitigating properties. Notably, caffeine's anti-adipogenic potential, attributed to the downregulation of key adipogenesis regulators, has been established. Recent findings further indicate that caffeine's influence on obesity may be mediated through alterations in the gut microbiota and its metabolic byproducts. Therefore, the present review summarizes the anti-adipogenic effect of caffeine in modulating obesity through the intestinal microbiota and its metabolites.
Keywords: anti-adipogenic effects; caffeine; gut microbiota; intestinal metabolites; metabolic insights; obesity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Landsberg L., Aronne L.J., Beilin L.J., Burke V., Igel L.I., Lloyd-Jones D., Sowers J. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment: A position paper of the Obesity Society and the American Society of Hypertension. J. Clin. Hypertens. 2013;15:14–33. doi: 10.1111/jch.12049. - DOI - PMC - PubMed
-
- Fox C.S., Massaro J.M., Hoffmann U., Pou K.M., Maurovich-Horvat P., Liu C.Y., Vasan R.S., Murabito J.M., Meigs J.B., Cupples L.A., et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

