Investigating the Role of 17-Beta Estradiol in the Regulation of the Unfolded Protein Response (UPR) in Pancreatic Beta Cells
- PMID: 38339098
- PMCID: PMC10855194
- DOI: 10.3390/ijms25031816
Investigating the Role of 17-Beta Estradiol in the Regulation of the Unfolded Protein Response (UPR) in Pancreatic Beta Cells
Abstract
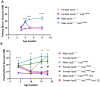
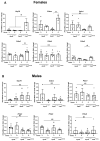
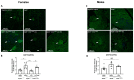
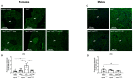
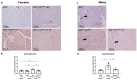
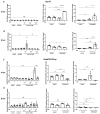
Diabetes mellitus is clinically defined by chronic hyperglycemia. Sex differences in the presentation and outcome of diabetes exist with premenopausal women having a reduced risk of developing diabetes, relative to men, or women after menopause. Accumulating evidence shows a protective role of estrogens, specifically 17-beta estradiol, in the maintenance of pancreatic beta cell health; however, the mechanisms underlying this protection are still unknown. To elucidate these potential mechanisms, we used a pancreatic beta cell line (BTC6) and a mouse model of hyperglycemia-induced atherosclerosis, the ApoE-/-:Ins2+/Akita mouse, exhibiting sexual dimorphism in glucose regulation. In this study we hypothesize that 17-beta estradiol protects pancreatic beta cells by modulating the unfolded protein response (UPR) in response to endoplasmic reticulum (ER) stress. We observed that ovariectomized female and male ApoE-/-:Ins2+/Akita mice show significantly increased expression of apoptotic UPR markers. Sham operated female and ovariectomized female ApoE-/-:Ins2+/Akita mice supplemented with exogenous 17-beta estradiol increased the expression of adaptive UPR markers compared to non-supplemented ovariectomized female ApoE-/-:Ins2+/Akita mice. These findings were consistent to what was observed in cultured BTC6 cells, suggesting that 17-beta estradiol may protect pancreatic beta cells by repressing the apoptotic UPR and enhancing the adaptive UPR activation in response to pancreatic ER stress.
Keywords: 17-beta estradiol; diabetes; endoplasmic reticulum stress; pancreatic beta cells; unfolded protein response.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Investigating the protective effects of estrogen on β-cell health and the progression of hyperglycemia-induced atherosclerosis.Am J Physiol Endocrinol Metab. 2022 Sep 1;323(3):E254-E266. doi: 10.1152/ajpendo.00353.2021. Epub 2022 Jul 13. Am J Physiol Endocrinol Metab. 2022. PMID: 35830687
-
Sex-Specific Differences in an ApoE(-/-):Ins2(+/Akita) Mouse Model of Accelerated Atherosclerosis.Am J Pathol. 2016 Jan;186(1):67-77. doi: 10.1016/j.ajpath.2015.09.009. Epub 2015 Nov 18. Am J Pathol. 2016. PMID: 26597883
-
PLIN2 is a Key Regulator of the Unfolded Protein Response and Endoplasmic Reticulum Stress Resolution in Pancreatic β Cells.Sci Rep. 2017 Jan 19;7:40855. doi: 10.1038/srep40855. Sci Rep. 2017. PMID: 28102311 Free PMC article.
-
Endoplasmic reticulum stress, degeneration of pancreatic islet β-cells, and therapeutic modulation of the unfolded protein response in diabetes.Mol Metab. 2019 Sep;27S(Suppl):S60-S68. doi: 10.1016/j.molmet.2019.06.012. Mol Metab. 2019. PMID: 31500832 Free PMC article. Review.
-
Beta cell endoplasmic reticulum stress drives diabetes in the KINGS mouse without causing mass beta cell loss.Diabet Med. 2022 Dec;39(12):e14962. doi: 10.1111/dme.14962. Epub 2022 Oct 9. Diabet Med. 2022. PMID: 36151994 Free PMC article. Review.
Cited by
-
The Role of Estrogen and ER Stress in Glycemic Regulation in the Sexually Dimorphic TALLYHO/JngJ Mouse Model of Diabetes.J Endocr Soc. 2025 Mar 15;9(5):bvaf048. doi: 10.1210/jendso/bvaf048. eCollection 2025 May. J Endocr Soc. 2025. PMID: 40191018 Free PMC article.
-
The Role of Estrogen across Multiple Disease Mechanisms.Curr Issues Mol Biol. 2024 Jul 29;46(8):8170-8196. doi: 10.3390/cimb46080483. Curr Issues Mol Biol. 2024. PMID: 39194700 Free PMC article. Review.
References
-
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C., Basit A., Chan J.C., Mbanya J.C., et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

