Molecular and Structural Alterations of Skeletal Muscle Tissue Nuclei during Aging
- PMID: 38339110
- PMCID: PMC10855217
- DOI: 10.3390/ijms25031833
Molecular and Structural Alterations of Skeletal Muscle Tissue Nuclei during Aging
Abstract
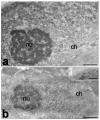
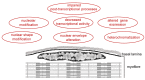
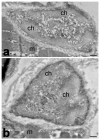
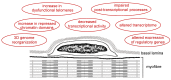
Aging is accompanied by a progressive loss of skeletal muscle mass and strength. The mechanisms underlying this phenomenon are certainly multifactorial and still remain to be fully elucidated. Changes in the cell nucleus structure and function have been considered among the possible contributing causes. This review offers an overview of the current knowledge on skeletal muscle nuclei in aging, focusing on the impairment of nuclear pathways potentially involved in age-related muscle decline. In skeletal muscle two types of cells are present: fiber cells, constituting the contractile muscle mass and containing hundreds of myonuclei, and the satellite cells, i.e., the myogenic mononuclear stem cells occurring at the periphery of the fibers and responsible for muscle growth and repair. Research conducted on different experimental models and with different methodological approaches demonstrated that both the myonuclei and satellite cell nuclei of aged skeletal muscles undergo several structural and molecular alterations, affecting chromatin organization, gene expression, and transcriptional and post-transcriptional activities. These alterations play a key role in the impairment of muscle fiber homeostasis and regeneration, thus contributing to the age-related decrease in skeletal muscle mass and function.
Keywords: RNA processing; cell nucleus; chromatin; gene expression; myofiber; ribonucleic acid (RNA) transcription; satellite cell; skeletal muscle atrophy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

