Exploring Novel Frontiers: Leveraging STAT3 Signaling for Advanced Cancer Therapeutics
- PMID: 38339245
- PMCID: PMC10854592
- DOI: 10.3390/cancers16030492
Exploring Novel Frontiers: Leveraging STAT3 Signaling for Advanced Cancer Therapeutics
Abstract
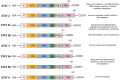
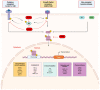
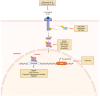
Signal Transducer and Activator of Transcription 3 (STAT3) plays a significant role in diverse physiologic processes, including cell proliferation, differentiation, angiogenesis, and survival. STAT3 activation via phosphorylation of tyrosine and serine residues is a complex and tightly regulated process initiated by upstream signaling pathways with ligand binding to receptor and non-receptor-linked kinases. Through downstream deregulation of target genes, aberrations in STAT3 activation are implicated in tumorigenesis, metastasis, and recurrence in multiple cancers. While there have been extensive efforts to develop direct and indirect STAT3 inhibitors using novel drugs as a therapeutic strategy, direct clinical application remains in evolution. In this review, we outline the mechanisms of STAT3 activation, the resulting downstream effects in physiologic and malignant settings, and therapeutic strategies for targeting STAT3. We also summarize the pre-clinical and clinical evidence of novel drug therapies targeting STAT3 and discuss the challenges of establishing their therapeutic efficacy in the current clinical landscape.
Keywords: STAT3; cancer; therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

