Effects of Ionizing Radiation on Cardiac Implantable Electronic Devices (CIEDs) in Patients with Esophageal Cancer Undergoing Radiotherapy: A Pilot Study
- PMID: 38339306
- PMCID: PMC10854512
- DOI: 10.3390/cancers16030555
Effects of Ionizing Radiation on Cardiac Implantable Electronic Devices (CIEDs) in Patients with Esophageal Cancer Undergoing Radiotherapy: A Pilot Study
Abstract
(1) Background: The prevalence of cancer patients relying on cardiac implantable electronic device (CIED) is steadily rising. The aim of this study was to evaluate RT-related malfunctions of CIEDs. (2) Methods: We retrospectively analyze sixteen patients with esophageal cancer who were treated with radiotherapy between 2012 and 2022 at the University Hospital Heidelberg. All patients underwent systemic evaluation including pre-therapeutic cardiological examinations of the CIED functionality and after every single irradiation. (3) Results: Sixteen patients, predominantly male (14) with a mean age of 77 (range: 56-85) years were enrolled. All patients received 28 fractions of radiotherapy with a cumulative total dose 58.8 Gy. The mean maximum dose at the CIEDs was 1.8 Gy. Following radiotherapy and during the one-year post-radiation follow-up period, there were no registered events associated with the treatment in this evaluation. (4) Conclusion: The study did not observe any severe CIED malfunctions following each radiation fraction or after completion of RT. Strict selection of photon energy and alignment with manufacturer-recommended dose limits appear to be important. Our study showed no major differences in the measured values of the pacing threshold, sensing threshold and lead impedance after RT.
Keywords: cardiac devices; esophageal cancer; radiotherapy; toxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
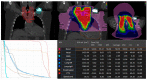
Figures
Similar articles
-
Impact of Radiotherapy on Malfunctions and Battery Life of Cardiac Implantable Electronic Devices in Cancer Patients.Cancers (Basel). 2023 Oct 2;15(19):4830. doi: 10.3390/cancers15194830. Cancers (Basel). 2023. PMID: 37835524 Free PMC article.
-
Radiotherapy-induced malfunctions of cardiac implantable electronic devices in cancer patients.Intern Emerg Med. 2020 Sep;15(6):967-973. doi: 10.1007/s11739-019-02240-y. Epub 2019 Dec 2. Intern Emerg Med. 2020. PMID: 31792775
-
Safety of lung stereotactic ablative radiotherapy for the functioning of cardiac implantable electronic devices.Radiother Oncol. 2021 Mar;156:193-198. doi: 10.1016/j.radonc.2020.12.029. Epub 2020 Dec 30. Radiother Oncol. 2021. PMID: 33387584
-
A systematic review and meta-analysis on oncological radiotherapy in patients with a cardiac implantable electronic device: Prevalence and predictors of device malfunction in 3121 patients.Eur J Clin Invest. 2023 Jan;53(1):e13862. doi: 10.1111/eci.13862. Epub 2022 Sep 7. Eur J Clin Invest. 2023. PMID: 36004486 Free PMC article.
-
Risk of cardiac implantable device malfunction in cancer patients receiving proton therapy: an overview.Front Oncol. 2023 Jul 4;13:1181450. doi: 10.3389/fonc.2023.1181450. eCollection 2023. Front Oncol. 2023. PMID: 37469405 Free PMC article. Review.
References
-
- Mond H.G., Irwin M., Ector H., Proclemer A. The world survey of cardiac pacing and cardioverter-defibrillators: Calendar year 2005 an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin. Electrophysiol. 2008;31:1202–1212. doi: 10.1111/j.1540-8159.2008.01164.x. - DOI - PubMed
-
- Hashii H., Hashimoto T., Okawa A., Shida K., Isobe T., Hanmura M., Nishimura T., Aonuma K., Sakae T., Sakurai H. Comparison of the effects of high-energy photon beam irradiation (10 and 18 MV) on 2 types of implantable cardioverter-defibrillators. Int. J. Radiat. Oncol. Biol. Phys. 2013;85:840–845. doi: 10.1016/j.ijrobp.2012.05.043. - DOI - PubMed
LinkOut - more resources
Full Text Sources

