Keeping Pathologists in the Loop and an Adaptive F1-Score Threshold Method for Mitosis Detection in Canine Perivascular Wall Tumours
- PMID: 38339394
- PMCID: PMC10854568
- DOI: 10.3390/cancers16030644
Keeping Pathologists in the Loop and an Adaptive F1-Score Threshold Method for Mitosis Detection in Canine Perivascular Wall Tumours
Abstract
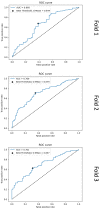
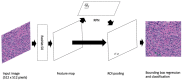
Performing a mitosis count (MC) is the diagnostic task of histologically grading canine Soft Tissue Sarcoma (cSTS). However, mitosis count is subject to inter- and intra-observer variability. Deep learning models can offer a standardisation in the process of MC used to histologically grade canine Soft Tissue Sarcomas. Subsequently, the focus of this study was mitosis detection in canine Perivascular Wall Tumours (cPWTs). Generating mitosis annotations is a long and arduous process open to inter-observer variability. Therefore, by keeping pathologists in the loop, a two-step annotation process was performed where a pre-trained Faster R-CNN model was trained on initial annotations provided by veterinary pathologists. The pathologists reviewed the output false positive mitosis candidates and determined whether these were overlooked candidates, thus updating the dataset. Faster R-CNN was then trained on this updated dataset. An optimal decision threshold was applied to maximise the F1-score predetermined using the validation set and produced our best F1-score of 0.75, which is competitive with the state of the art in the canine mitosis domain.
Keywords: artificial intelligence; canine Perivascular Wall Tumour; canine Soft Tissue Sarcoma; deep learning; digital pathology; faster R-CNN; humans in the loop; mitosis; mitosis detection; object detection; pathologists in the loop.
Conflict of interest statement
Zoetis funded part of the original study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Cavalcanti E.B., Gorza L.L., de Sena B.V., Sossai B.G., Junior M.C., Flecher M.C., Marcolongo-Pereira C., dos Santos Horta R. Correlation of Clinical, Histopathological and Histomorphometric Features of Canine Soft Tissue Sarcomas. Braz. J. Vet. Pathol. 2021;14:151–158. doi: 10.24070/bjvp.1983-0246.v14i3p151-158. - DOI
LinkOut - more resources
Full Text Sources

